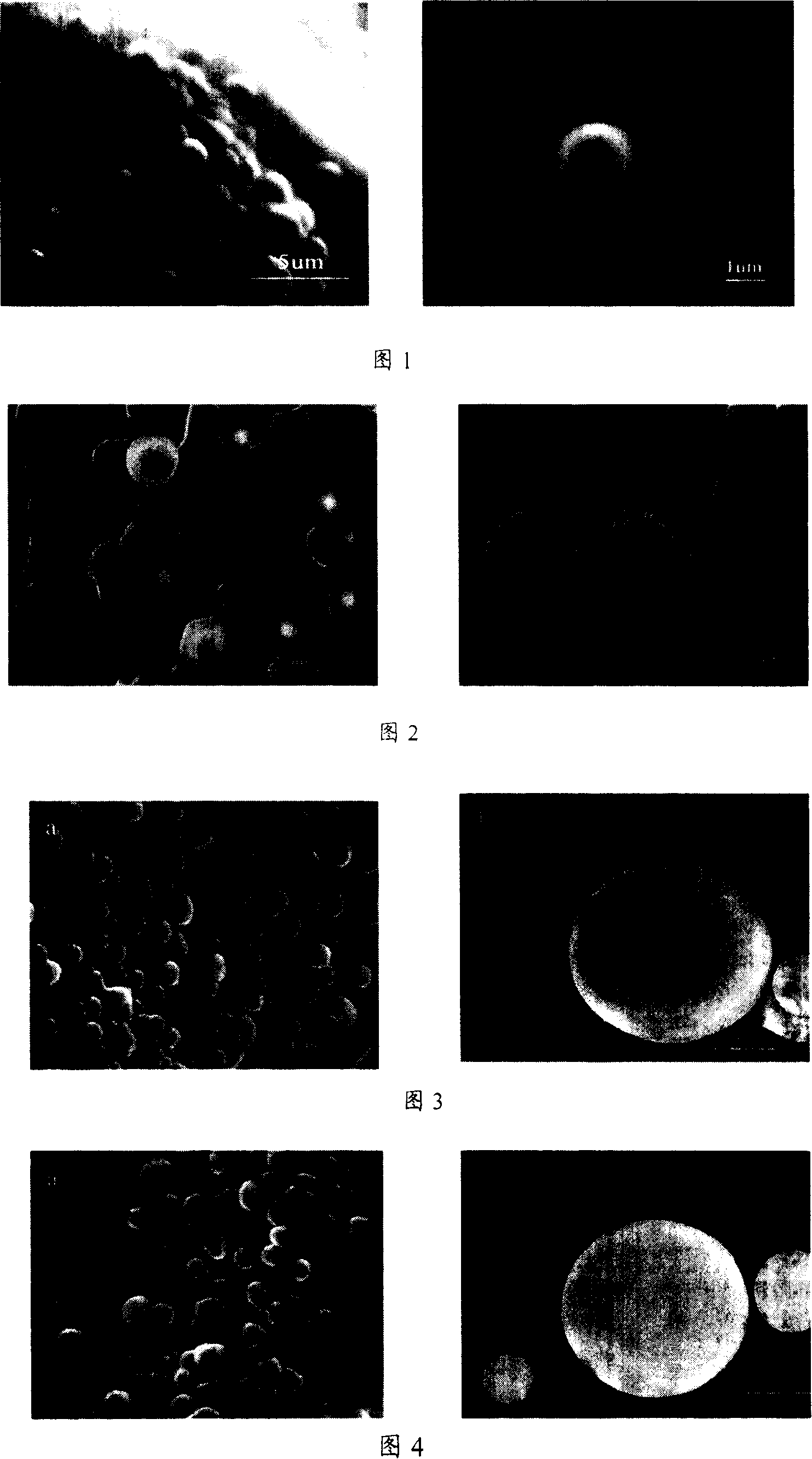
Preparation for modified chitosan metal complexes micro-sphere used for carrying medicament and preparation for the modified chitosan
A chitosan derivative and chitosan technology, applied in the field of medicine, can solve problems such as poor solubility, low biological activity, and limited application range, and achieve the effects of low cost, good slow-release performance, and increased solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
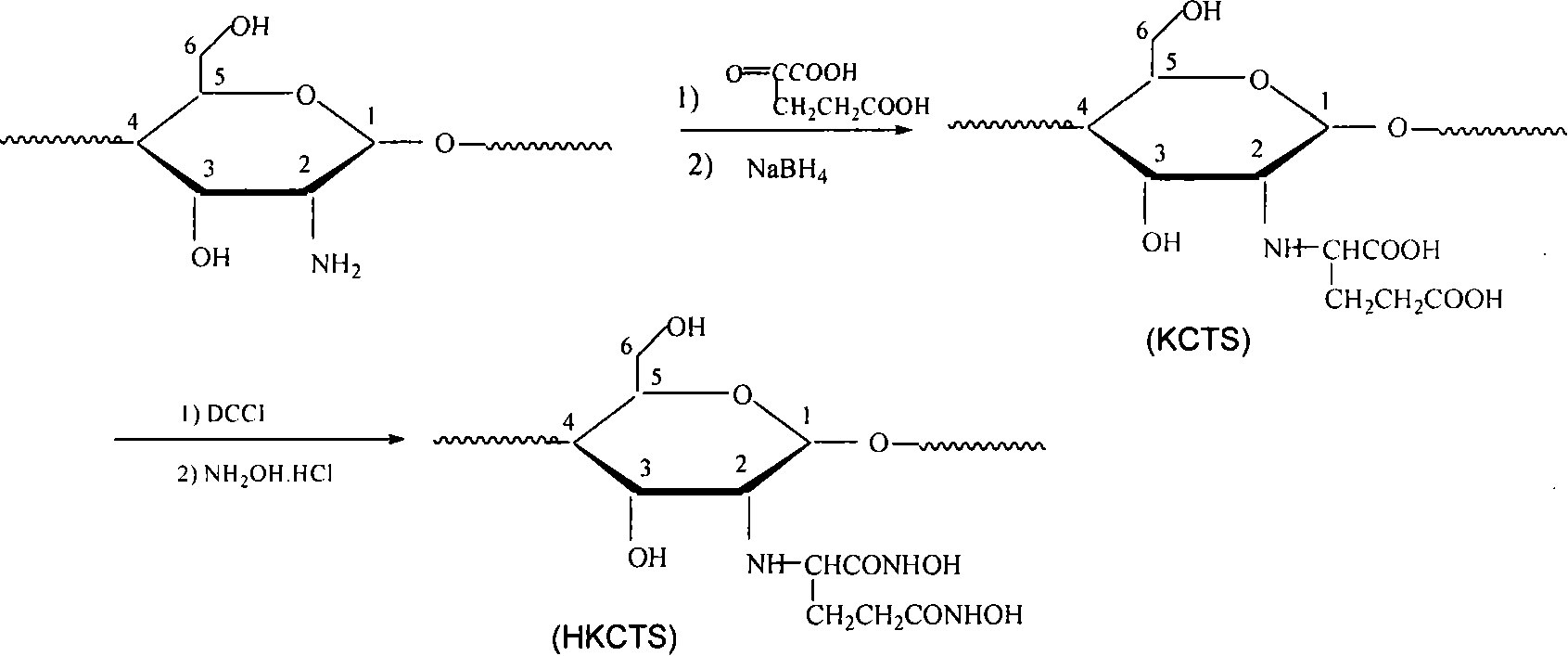
[0030] The preparation of modified chitosan α-ketoglutaric acid chitosan (KCTS) is as follows: weigh chitosan (CTS) dry sample, fully swell with distilled water, add α-ketoglutaric acid, filter, and use NaOH solution Adjust the pH to 4-5, continue the reaction in a water bath at 35-38°C, add sodium borohydride, adjust the pH to 6.5-7.0 with dilute HCl solution, continue the reaction for 20-28 hours, pour the reaction mixture into 90-97% ethanol to terminate the reaction , KCTS was precipitated, filtered under reduced pressure, washed, the product was transferred to a Soxhlet extractor for continuous extraction for 6-9 hours, and dried to obtain a white powdery solid α-ketoglutarate chitosan (KCTS).
[0031] Using chitosan molecules on C 2 Active free amino-NH 2 The functional polymer α-ketoglutarate chitosan (KCTS) with Schiff base groups can be synthesized by macromolecular reaction with α-ketoglutarate. The prepared KCTS has improved solubility, has special chelating effec...
Embodiment 2
[0033]The preparation of modified chitosan hydroxylamine α-ketoglutaric acid chitosan (HKCTS) is: 0.5-1.0g KCTS is dissolved in water, and the pH value of polymer solution is adjusted to 4.0- with 0.1mol / L hydrochloric acid solution 5.0 Slowly add 0.74-1.48g of dicyclohexylcarbodiimide (DCCI) to the mixture under stirring, after 2-3h of reaction, add 5.0-10.0g of hydroxylamine hydrochloride, and further react for 1h under stirring; then use sodium hydroxide Adjust the pH value to 9.0-10.0, and stir at room temperature for more than 20-28 hours until the reaction mixture appears HKCTS precipitation in the mixture of 10-20ml concentrated hydrochloric acid and 250-300ml acetone to stop the reaction. Filtration and washing were carried out, and the resulting polymer was dried overnight at room temperature.
[0034] The solubility of the prepared HKCTS is greatly improved, and can be completely dissolved in water, soluble in 2% NaOH and 10% ammonia water. HKCTS introduces -CONHOH ...
Embodiment 3
[0036] (1) Accurately weigh 1.000 g of KCTS (preparation), add it to 20.00 mL of 0.1 mol / L NaOH solution, fully swell or dissolve for 30 min, and stir evenly;
[0037] (2) Accurately weigh 1.000 g of theophylline for oral administration, add it to the above system, and stir at a constant temperature of 40° C. for 1 hour to obtain a milky white viscous polymer solution;
[0038] (3), accurately weigh 1.8gZnSO 4 ·7H 2 O made into ZnSO with a concentration of 18mg / mL 4 ·7H 2 O aqueous solution 100.00mL;
[0039] (4), slowly add the milky white viscous polymer solution to 18mg / mL ZnCl 2 In 100.00mL of aqueous solution, adjust the pH of the system to about 6, keep stirring and reacting at a constant temperature in a water bath for more than 9 hours, and a large number of white polymer microspheres are formed;
[0040] (5), carry out suction filtration under reduced pressure, and the filtrate is washed 2-3 times with distilled water and a small amount of absolute ethanol succes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com