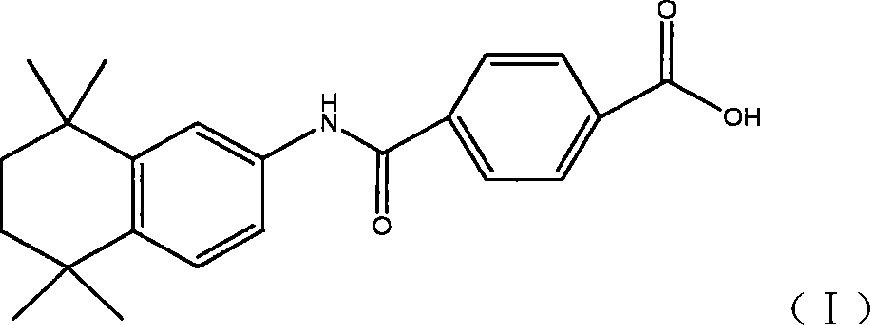
Synthetic technique for tamibarotene
A technology of tamibarotene and a compound, which is applied in the field of new synthesis process of tamibarotene, can solve the problems of complicated operation, long synthesis route, low yield and the like, and achieves the effect of simplifying the synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] The preparation of example one 2,5-dichloro-2,5-dimethylhexane
[0033] Add 24 grams of 2,5-dimethyl-2,5-hexanediol into 400ml of concentrated hydrochloric acid, stir at room temperature for 2 hours, filter out the solid with suction, dissolve it in 50ml of dichloromethane, wash with water until neutral, and anhydrous Dry over magnesium sulfate, filter, and concentrate under reduced pressure to obtain 24.4 g of a white solid with a yield of 81% and a melting point of 65-66°C.
example 2
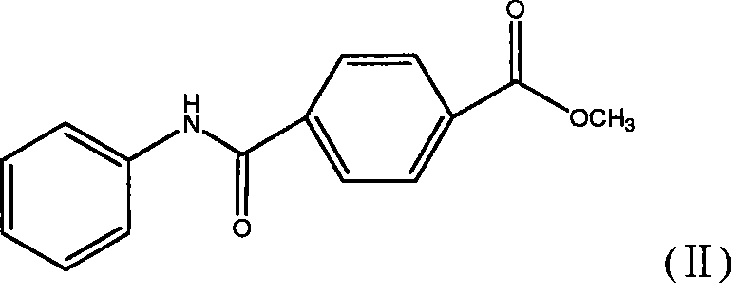
[0034] The preparation of example two p-phenylcarbamoylbenzoic acid methyl esters (II)
[0035] Dissolve 24.4g of methyl p-chloroformylbenzoate in 200ml of anhydrous dichloromethane, then add 25ml of triethylamine, then slowly add 30ml of aniline, keep the internal temperature at 0-5°C, and stir at room temperature after the dropwise addition After 2 hours, the solid was filtered out with suction, washed with water until neutral, and dried to obtain a light yellow crude product, which was recrystallized from ethanol to obtain 27.3 g of off-white solid, yield 87%, melting point: 194°C-196°C.
[0036] 1 H-NMR (CDCl 3 )δ: 3.95 (3H, s, CH 3 ); 7.19 (1H, t, aromatic H); 7.38 (2H, t, aromatic H); 7.64 (2H, d, aromatic H); 7.91 (2H, d, aromatic H); 7.97 (1H, s, NH) ; 8.12 (2H, d, aromatic H).
example 3 -
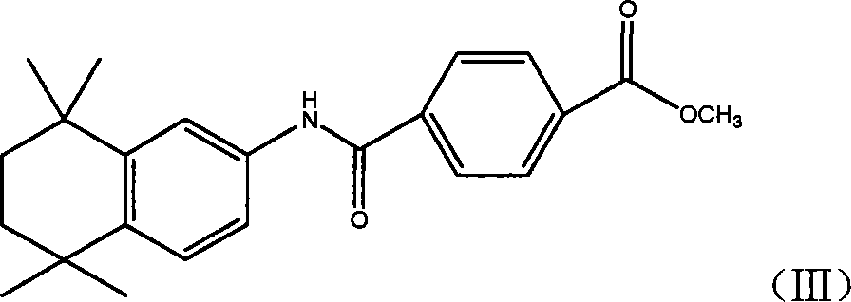
[0037] Preparation of Example Three 4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalene)carbamoyl]benzoic acid methyl ester (III)
[0038] Add 27.2 grams of compound (II) into 200 ml of anhydrous dichloromethane, then add 27 grams of anhydrous aluminum trichloride and 35 grams of 2,5-dichloro-2,5-dimethylhexane in sequence, and keep the internal temperature Stir for 2 hours at -5 to -10°C. Then the reactant was slowly poured into 250ml of ice water, the organic layer was separated, the aqueous layer was extracted with dichloromethane (60ml×2), the organic layers were combined, washed with water until neutral, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain The tan residue was recrystallized from n-hexane to obtain a dark yellow solid, and then recrystallized from a methanol / water mixed solvent to obtain 20 g of a light yellow solid with a yield of 52%. Melting point: 212°C-215°C.
[0039] 1 H-NMR (CDCl 3 )δ: 1.28 (6H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com