Method for preparing fluoorpropane and device thereof
A technology for preparing fluoropropane and equipment, which is applied in the field of fluoropropane preparation, and can solve the problems of increasing input volume, difficulty in separation, and high requirements for equipment preparation, and achieve the effects of reducing side reactions, increasing crude product content, and improving activation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
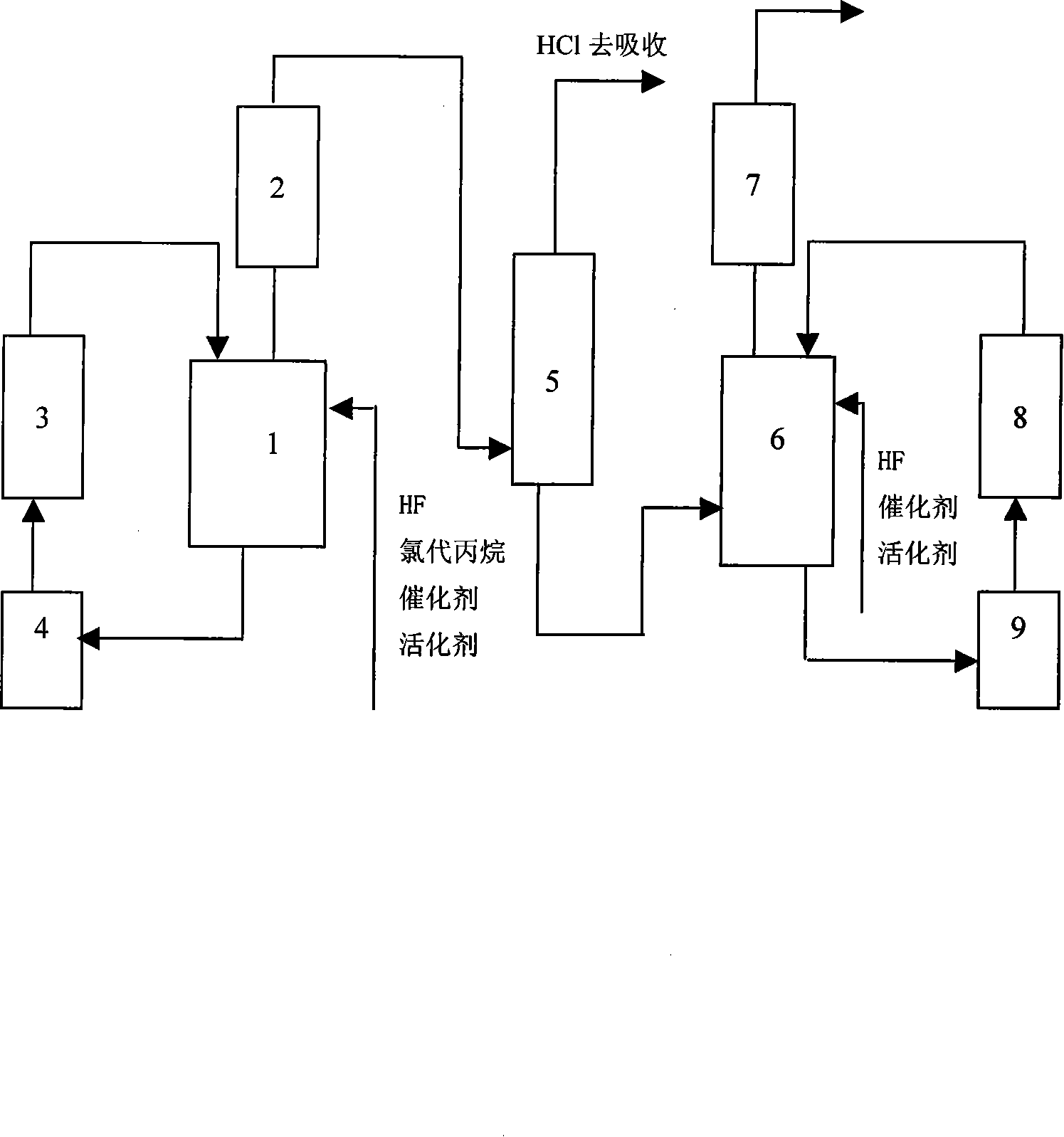
[0013] The preparation method of fluoropropane is to add chloropropane, anhydrous hydrofluoric acid, and catalyst in the primary fluorination reactor 1 with a molar ratio of 1:3~10:0.05~5, and the materials in the reactor are passed through a The first-stage circulation pump 4 and the first-stage heat exchanger 3 carry out a circulation reaction, and the reaction products containing hydrogen chloride, fluoropropane, intermediates and hydrogen fluoride are ejected from the reflux tower 2, and hydrogen chloride is separated and removed by the hydrogen chloride separation tower 5, and the remaining reaction products Enter the secondary fluorination reactor 6, the tower kettle material in the secondary fluorination reactor 6 passes through the secondary circulation pump 9, the secondary heat exchanger 8, and carries out a circulation reaction, containing fluoropropane, hydrogen chloride, intermediates and hydrogen fluoride The reaction product comes out of the secondary reflux towe...
Embodiment 1
[0016] Add chloropropane, anhydrous hydrofluoric acid, and catalyst with a molar ratio of 1:3:0.05 into the primary fluorination reactor 1, and the materials in the tower tank in the reactor pass through the primary circulation pump 4 and the primary heat exchanger 3 , carry out circulation reaction, the reaction product containing hydrogen chloride, fluoropropane, intermediate and hydrogen fluoride is ejected from reflux tower 2, is separated and removed hydrogen chloride by hydrogen chloride separation tower 5, and remaining reaction product enters secondary fluorination reactor 6, secondary The materials in the tower kettle in the fluorination reactor 6 are circulated through the secondary circulation pump 9 and the secondary heat exchanger 8, and the reaction products containing fluoropropane, hydrogen chloride, intermediates and hydrogen fluoride are ejected from the secondary reflux tower 7, After absorbing hydrogen chloride, washing with water and alkali, drying, and rec...
Embodiment 2
[0018] Add chloropropane, anhydrous hydrofluoric acid, and catalyst with a molar ratio of 1:10:5 into the primary fluorination reactor 1, and the materials in the tower tank in the reactor pass through the primary circulating pump 4 and the primary heat exchanger 3 , carry out circulation reaction, the reaction product containing hydrogen chloride, fluoropropane, intermediate and hydrogen fluoride is ejected from reflux tower 2, is separated and removed hydrogen chloride by hydrogen chloride separation tower 5, and remaining reaction product enters secondary fluorination reactor 6, secondary The materials in the tower kettle in the fluorination reactor 6 are circulated through the secondary circulation pump 9 and the secondary heat exchanger 8, and the reaction products containing fluoropropane, hydrogen chloride, intermediates and hydrogen fluoride are ejected from the secondary reflux tower 7, After absorbing hydrogen chloride, washing with water and alkali, drying, and recti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com