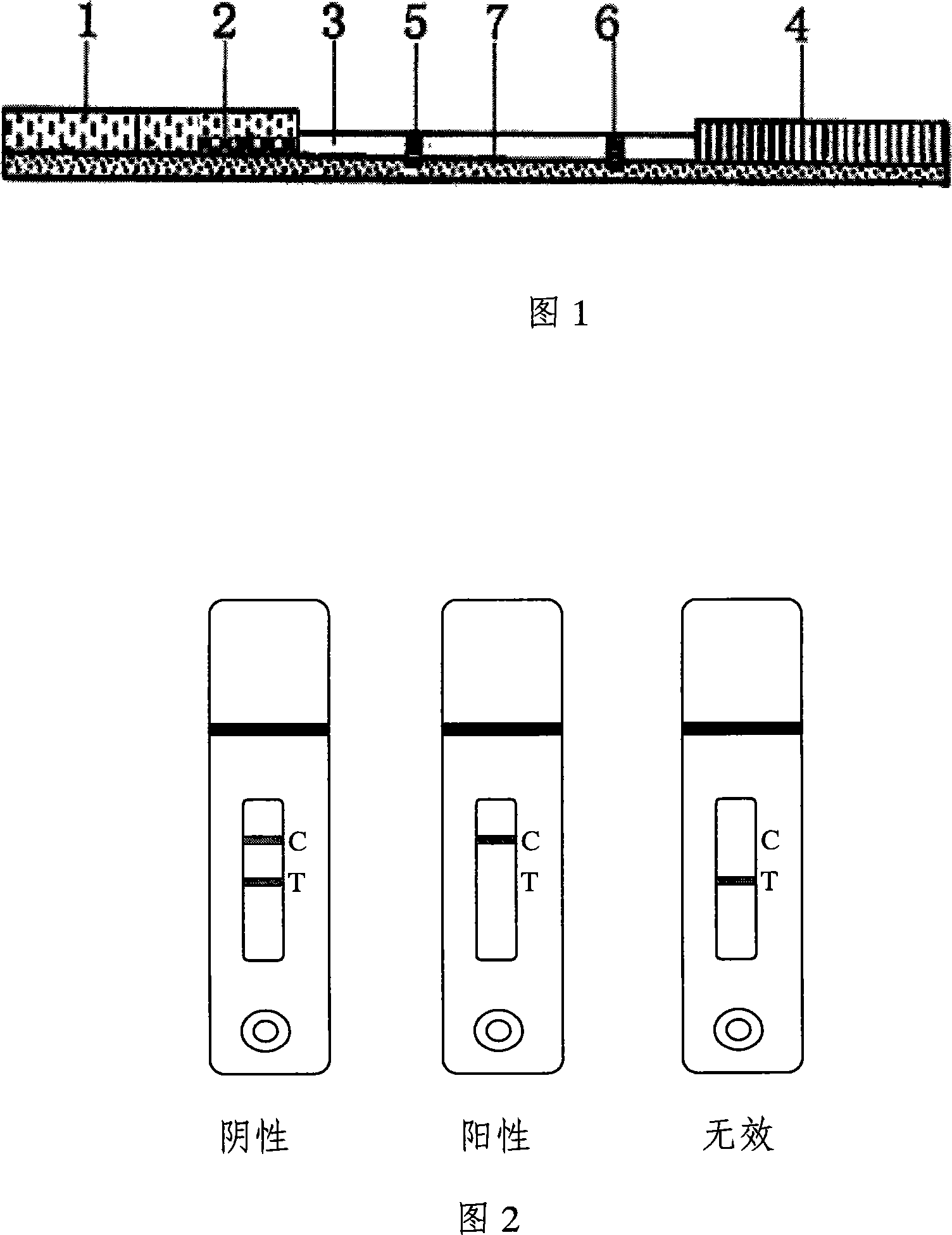
Colloidal gold test paper for detecting sulphaquinoxaline drug residue
The technology of sulfaquinoxaline and colloidal gold test paper is applied in the field of immunochemical rapid detection of veterinary drug residues, which can solve the problems of long detection time, high cost, unfavorable promotion and use of basic units, etc., and achieves short detection time, high sensitivity, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 SQX detects the preparation of test paper card
[0032] 1. Synthesis and identification of SQX-carrier protein conjugates
[0033] (1) Synthesis of SQX-human serum albumin (HSA) conjugate
[0034] Configure 4mol / L SQX with 0.1mol / L hydrochloric acid solution, add 1% NaNO dropwise 2 (excess), 4°C with constant stirring. NaNO 2 The amount of added starch can be monitored with starch-potassium iodide test paper or by adding 1% starch and 50mol / LKI to white tiles. Free nitrous acid can oxidize iodide to iodine. Iodine then reacts with starch to turn it dark blue. After the solution turned dark blue, the reaction was continued for 15 min. Human serum protein was dissolved with boric acid buffer at pH 8.6 and concentration 2.0 mol / L. Add the diazotized hapten (to prevent local acid excess) while stirring, and adjust the pH to 9.5. The reaction was stirred for 2 h in the refrigerator, and the pH was adjusted to 9.0. Dialyze with PBS for 2 days and store a...
Embodiment 3
[0062] Example 3 Detection of Residues in Samples
[0063] 1. Sample pretreatment
[0064] (1) Animal tissue (chicken, chicken liver, pork, pork liver) samples
[0065] Weigh 2.0±0.05g of the homogenized tissue sample into a centrifuge tube, and tightly cap the bottle. Place the centrifuge tube containing the sample in a slightly boiling water bath for 10 minutes, absorb more than three drops of the boiled solution and pour it into a 1.5ml centrifuge tube. If there is obvious yellow turbidity, please centrifuge, and use the supernatant as the sample solution to be tested.
[0066] (2) Urine sample processing
[0067] Urine sample: Generally, it can be tested directly. When the urine is turbid, filter it with filter paper, and then do spot testing.
[0068] (3) Honey sample processing
[0069] Weigh 2.0±0.05g of honey, put it in a clean container, and add 4ml of borate buffer for dilution.
[0070] 2. Detection with test paper card
[0071] Drop the sample into the hole ...
Embodiment 4
[0077] Example 4 sample detection example
[0078] Take 15 eggs, chicken, and honey samples known to contain SQX concentrations greater than 10ng / g, and 15 negative samples each, and repeat the test twice for each sample, and calculate the negative and positive rates. The results are shown in Table 1, Table 2 and Table 3.
[0079] Table 1 Negative and positive rate detection of egg samples
[0080]
[0081] Table 2 Detection of negative and positive rate of chicken samples
[0082]
[0083] Table 3 Negative and positive rate detection of honey samples
[0084]
[0085] The results show that there are 15 samples of eggs, chicken, and honey, and 15 samples of negative samples. Each sample is tested twice. The false positive rate in eggs is less than 7%. The positive coincidence rate is 100%, and the negative coincidence rate is 93%. %above. The positive and negative coincidence rate of chicken is 100%. The false positive rate in honey is le...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com