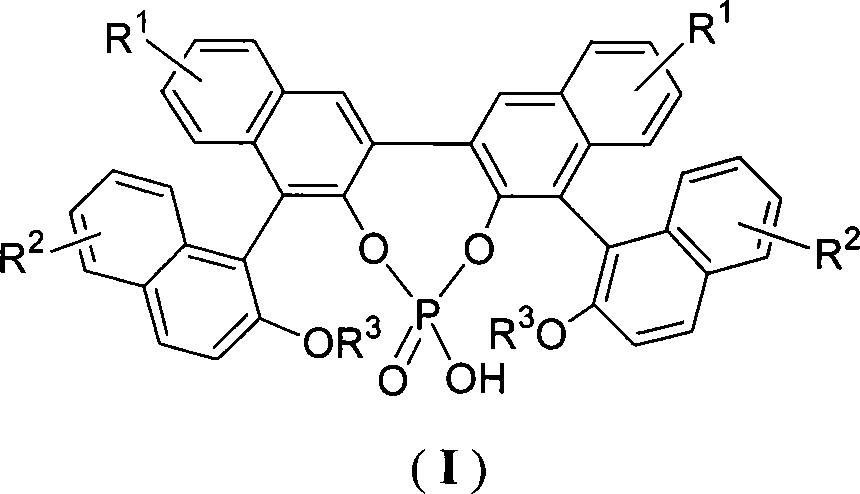
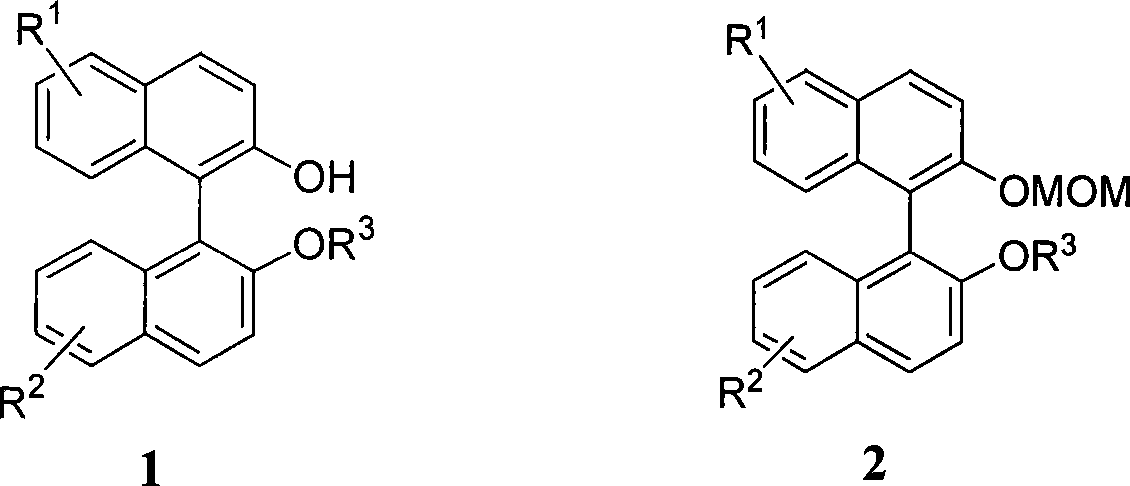
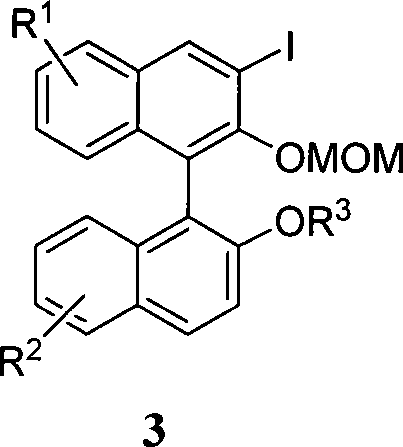
Organic phosphoric acid catalyst and its preparation method and application
An organophosphoric acid, organolithium technology, applied in organic chemistry methods, organic compound/hydride/coordination complex catalysts, organic chemistry and other directions, can solve the problem of large amount of catalyst used, achieve excellent catalytic performance, easy preparation, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of (R)-2'-cyclohexyloxy-(1,1'-binaphthyl)-2-ol
[0031]
[0032](R)-Binaphthol (BINOL) (8.58 g, 30 mmol), triphenylphosphine (7.86 g, 30 mmol) and cyclohexanol (9.0 g, 90 mmol) were dissolved in 60 mL of tetrahydrofuran. Under stirring at room temperature, a solution of diethyl azodicarboxylate (DEAD) (5.22 g, 30 mmol) in 40 mL of tetrahydrofuran was added dropwise to the above mixture over 4 hours. After stirring at room temperature for 20 hours, the mixture was evaporated to remove the solvent under reduced pressure. The residue was separated by silica gel column chromatography (petroleum ether-dichloromethane-ethyl acetate 40:10:1) to obtain oily (R)-2'-cyclohexyloxy-(1,1'-binaphthyl)-2- Phenol 4.3g, yield 39%. [α] D 20 =-55.0 (c1.0, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 ): δ0.93-1.64(m, 10H), 4.06-4.15(m, 1H), 5.14(s, 1H), 7.05-7.34(m, 8H), 7.74-7.81(m, 4H); 13 C NMR (75MHz, CDCl 3 ): δ22.9, 23.0, 25.2, 31.6, 76.9, 115.4, 117.2, 117.5,...
Embodiment 2
[0033] Example 2: Preparation of (R)-2-cyclohexyloxy-2'-methoxymethyl-1,1'-binaphthalene
[0034]
[0035] Dissolve (R)-2′-cyclohexyloxy-(1,1′-binaphthyl)-2-ol (9.74g, 26.47mmol) in 60mL tetrahydrofuran (THF), add 1.27g NaH under stirring at 0°C . The reaction mixture was stirred for an additional 1 h at 0°C and chloromethyl methyl ether (3.0 mL, 37 mmol) was added. As detected by TLC, the reaction was complete 6 hours after the addition of chloromethyl methyl ether. The reaction mixture was added to 100 ml of water, the aqueous phase was separated and extracted three times with ethyl acetate. The organic layer was washed with brine and water, followed by Na 2 SO 4 Dry and concentrate under reduced pressure. The crude product was separated by silica gel column chromatography, and the eluent was petroleum ether-dichloromethane-ethyl acetate (40:10:1) to obtain (R)-2-cyclohexyloxy-2'-methoxy 10.6 g of methyl-1,1'-binaphthalene, yield 97%. [α] D 20 =+64.1(c1.0, CHCl ...
Embodiment 3
[0036] Example 3: Preparation of (R)-2-methoxy-2'-methoxymethyl-3'-iodo-1,1'-binaphthalene
[0037]
[0038] Dissolve (R)-2-methoxy-2'-methoxymethyl-1,1'-binaphthyl (4.6g, 13.4mmol) in anhydrous tetrahydrofuran (100mL), and then add n- Butyllithium (16mmol, 7.0mL 2.3M hexane solution) was removed from the cooling bath and naturally raised to room temperature and stirred for 2 hours. A solution of iodine (4.57 g, 36 mmol) in tetrahydrofuran (20 mL) was added dropwise to the reaction mixture at -78°C, stirring was continued for 1 hour, and saturated Na 2 S 2 o 3 solution (30ml), then warmed to room temperature and stirred for 1 hour. The organic phase was separated and the aqueous phase was extracted with ethyl acetate (3 x 40 mL). The combined organic phases were washed with anhydrous NaSO 4 dry. Concentration under reduced pressure, the residue was separated by silica gel column chromatography, the eluent was petroleum ether-ethyl acetate (10:1), to obtain (R)-2-metho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com