Method for synthesizing polyhydroxy pyrroline acridine alkaloid
A technology of polyhydroxypyrrolidinine and alkaloids, which is applied in the field of synthesizing polyhydroxypyrrolidinium alkaloids to achieve high biological activity and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1, (3S, 4R, 5R)-3,4-2H-3,4-two-(benzyloxy)-5-benzyloxymethyl-2H-pyrrole 1-oxide (compound 2) synthesis
[0029]
[0030] To a solution of 2,3,5-tri-O-benzyl-ribofuranose (20 g, 47.6 mmol) in DCM (150 ml) was added pyridine (8 ml, 104.8 mmol) and NH 2 OH.HCl (3.7 g, 52.4 mmol). The reaction mixture was heated to reflux for 12 hours, cooled to room temperature, and diluted with DCM (200ml). The reaction mixture was washed with saturated brine (100ml×2), and washed with anhydrous Na 2 SO 4dry. After filtration, pyridine (8ml, 104.8mmol) and TBDMSCl (9g, 60mmol) were added to the filtrate. The reaction mixture was stirred at room temperature for 12 hours and then washed with saturated NaHCO 3 washed with aqueous solution, followed by anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate to a yellow oil. This crude product was dissolved in toluene (150ml), and PPh 3 (25.8g, 98.4mmol), imidazole (6.7g, 98.4mmol) and I 2 (25 g, 98.4 mmol). The rea...
Embodiment 2
[0034] Example 2: Synthesis of (2S, 3S, 4R, 5R)-N-hydroxyl-2-vinyl-3,4-bis-(benzyloxy)-5-benzyloxymethylpyrroline (compound 3)
[0035]
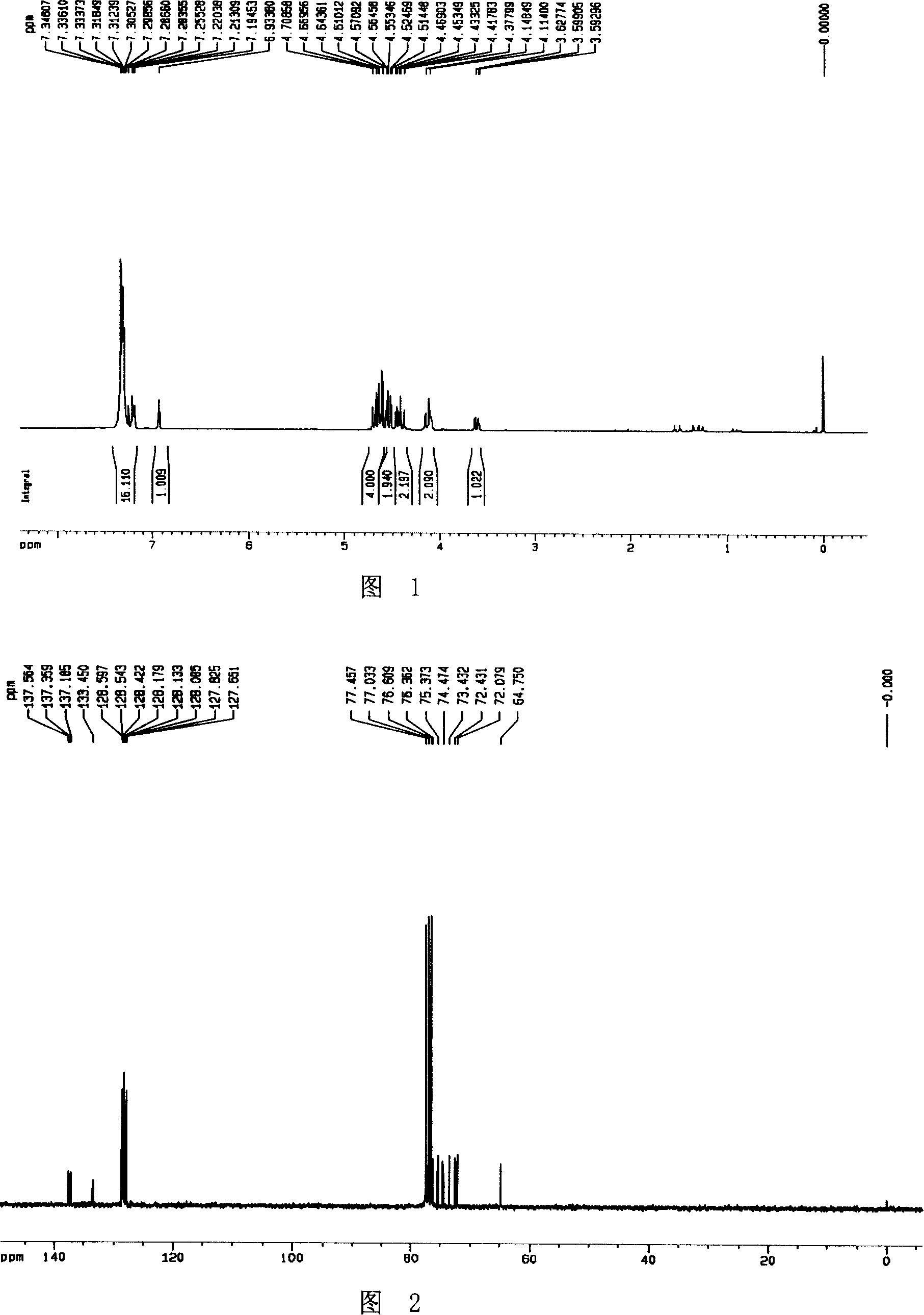
[0036] A THF (40ml) solution of nitrone compound 2 (1.8g, 4.3mmol) was cooled to -78°C, and a 1.6M THF solution of vinylmagnesium chloride (4.0ml, 6.4mmol) was added dropwise. The reaction mixture was stirred at -78°C for 0.5 h and then washed with saturated NH 4 Aqueous Cl solution quenched the reaction. The organic phase was separated, the aqueous phase was extracted with EtOAc (50ml×2), and the combined organic phases were washed with anhydrous Na 2 SO 4 dry. Column chromatography (PE:EA=5:1) gave compound 3 (1.8 g, 90%) as a white solid.
[0037] Its NMR H spectrum and C spectrum are shown in Figure 3 and Figure 4, respectively.
[0038] Mp 71-72°C; [α] D 20 -6.5 (c 2.15, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 )δ7.24-7.38 (15H, m, Ar-H), 5.89-5.79 (1H, m, CH 2 =CH), 5.45 (1H, dd, J=17.1, 0.9Hz, CH 2 =CH), 5.27 (1H, dd, J=10.2, ...
Embodiment 3
[0039] Example 3: Synthesis of (2S, 3S, 4R, 5R)-2-vinyl-3,4-bis-(benzyloxy)-5-benzyloxymethylpyrroline (compound 4)
[0040]
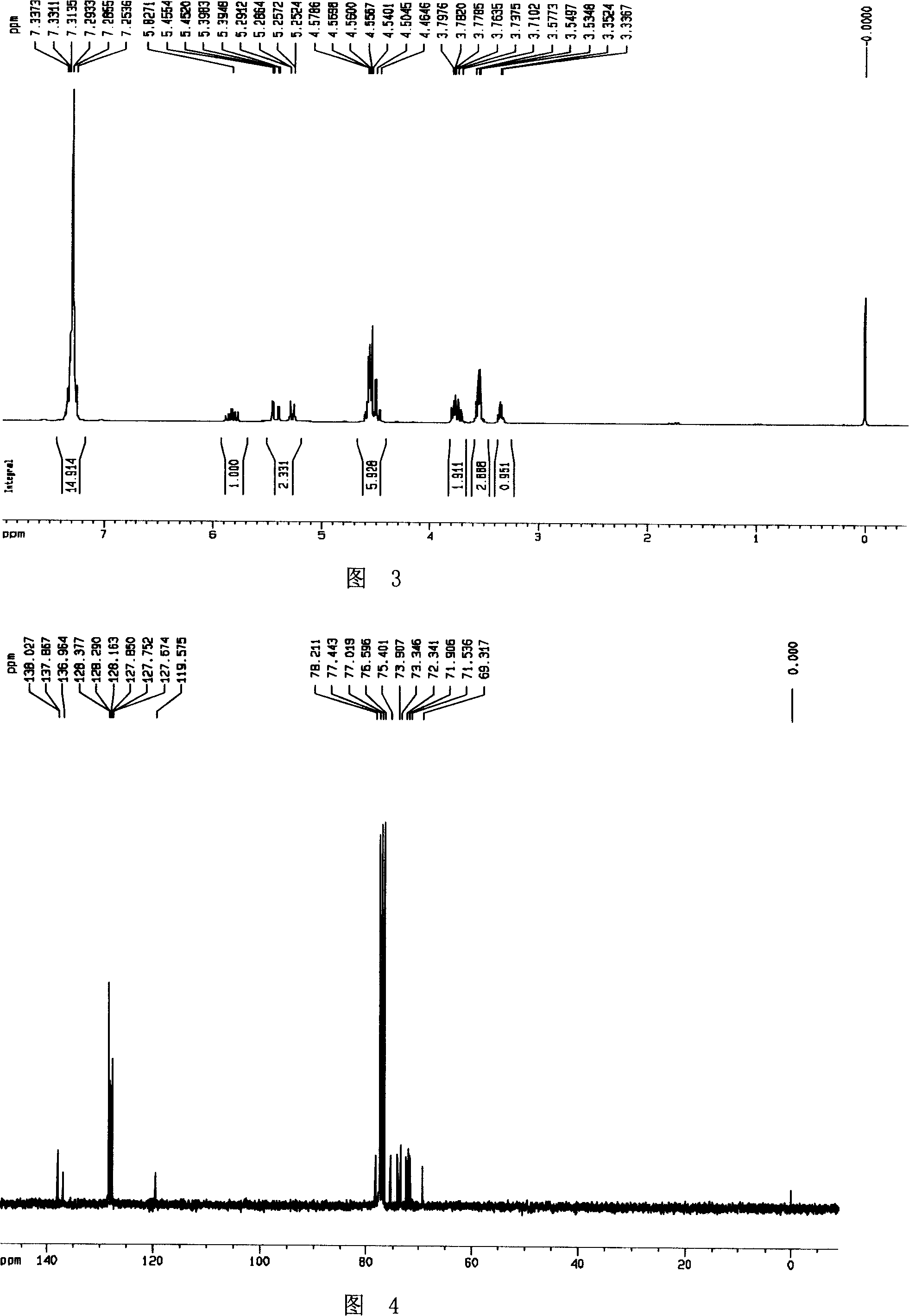
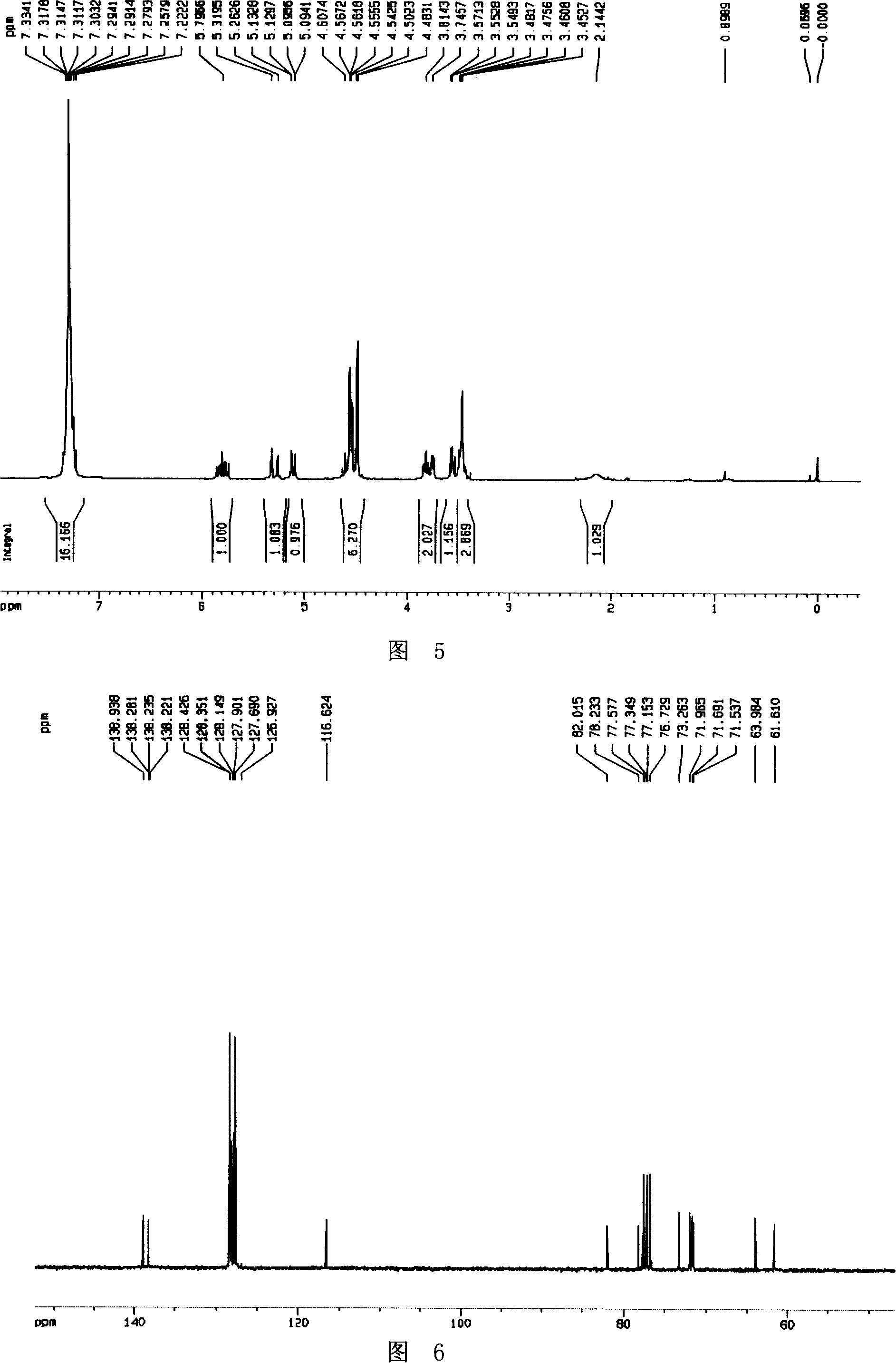
[0041] Cu(OAc) 2 ·H 2 O (86mg) was added to a suspension of zinc powder (880mg, 13.5mmol) and acetic acid (10ml), and the mixture was stirred at room temperature for 15 minutes, and then 20ml of hydroxylamine compound 3 (1.25g, 2.7mmol) was added in acetic acid-water ( 6:1, v:v) solution. The reaction mixture was heated to 70°C for half an hour. The solvent was distilled off under reduced pressure, and the aqueous phase was adjusted to pH=10 with 6N NaOH. Filtration, the filtrate was extracted with DCM (25ml×3), and the organic phases were combined with anhydrous Na 2 SO 4 dry. The crude product was separated by column chromatography (PE:EA=1:2) to obtain compound 4 (1.15 g, 95%) as a colorless oil.
[0042] Its NMR H spectrum and C spectrum are shown in Figure 5 and Figure 6, respectively.
[0043] [α] D 20 +11.9 (c 0.67, CHCl 3 ); 1 H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com