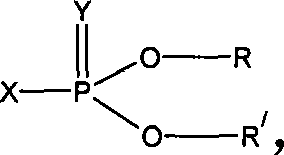
Method of synthesizing general 2-methoxy phosphoric acid ester pesticide hapten
A technology of dimethoxyphosphoric acid and synthesis method, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., to achieve the effect of improving detection sensitivity and reducing detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
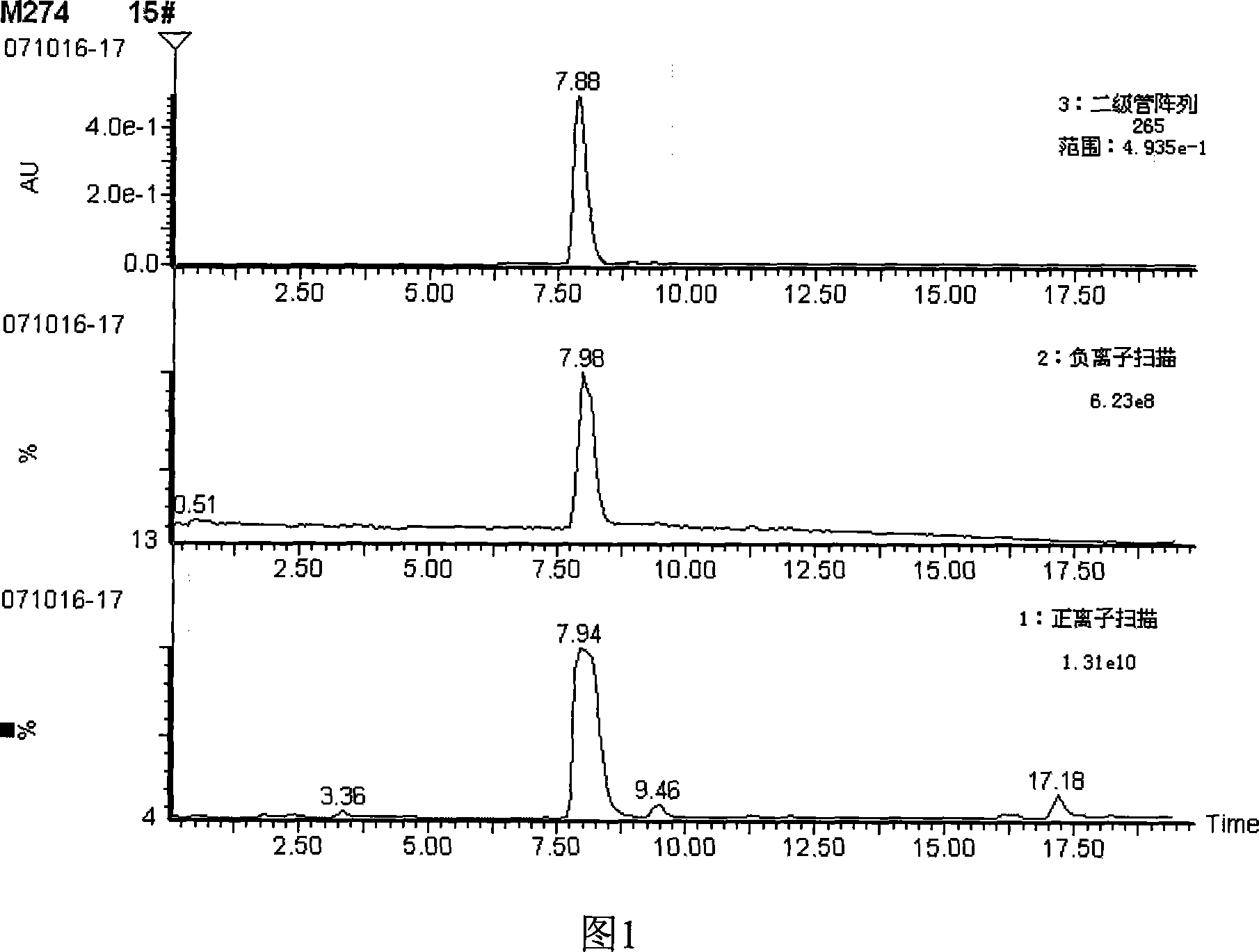
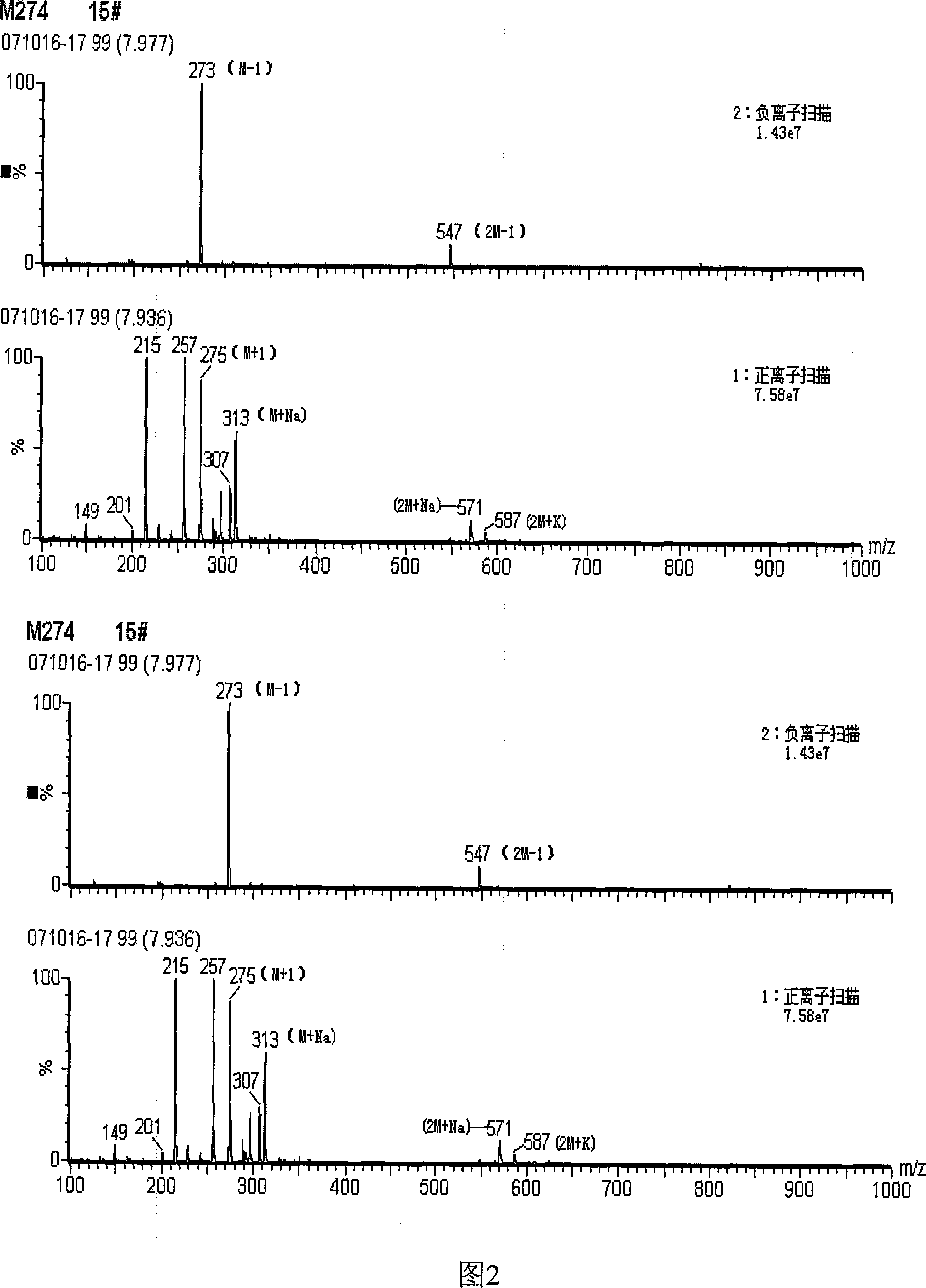
Image
Examples
Embodiment Construction
[0028] (1) Esterification
[0029] Add 3.5g (21mmol) of p-hydroxyphenylpropionic acid, 14mL of methanol and 1mL of concentrated sulfuric acid into a 50mL flask, and heat to reflux at 120°C for 2h. Afterwards, about 5mL of methanol was distilled off, and a saturated solution of sodium carbonate was added until no gas was generated. Then it was extracted with dichloromethane, the solvent was removed by rotary evaporation at 35°C, and the reddish-brown oil was obtained by vacuum drying. The purity of the product was over 99%.
[0030] (2) Acylation
[0031] In a 50mL round bottom flask, add 10-15mL of acetonitrile as a solvent, add 1.9280g (10mmoL) of the first product methyl p-hydroxyphenylpropionate, 10mmoL of dimethoxyphosphoryl chloride, and 2.5g of potassium carbonate, and stir magnetically at room temperature For about 2 hours, the progress of the reaction was continuously monitored by TLC during the reaction until the acid chloride was completely reacted. Then let it st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com