Method for preparing high-purity apolipoprotein A-I
An apolipoprotein and A-I technology, which is applied to the preparation field of apolipoprotein A-I, can solve the problems of unsuitable industrial production, low protein yield, flammability and explosion, etc., and is suitable for large-scale industrial production and has good protein activity. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
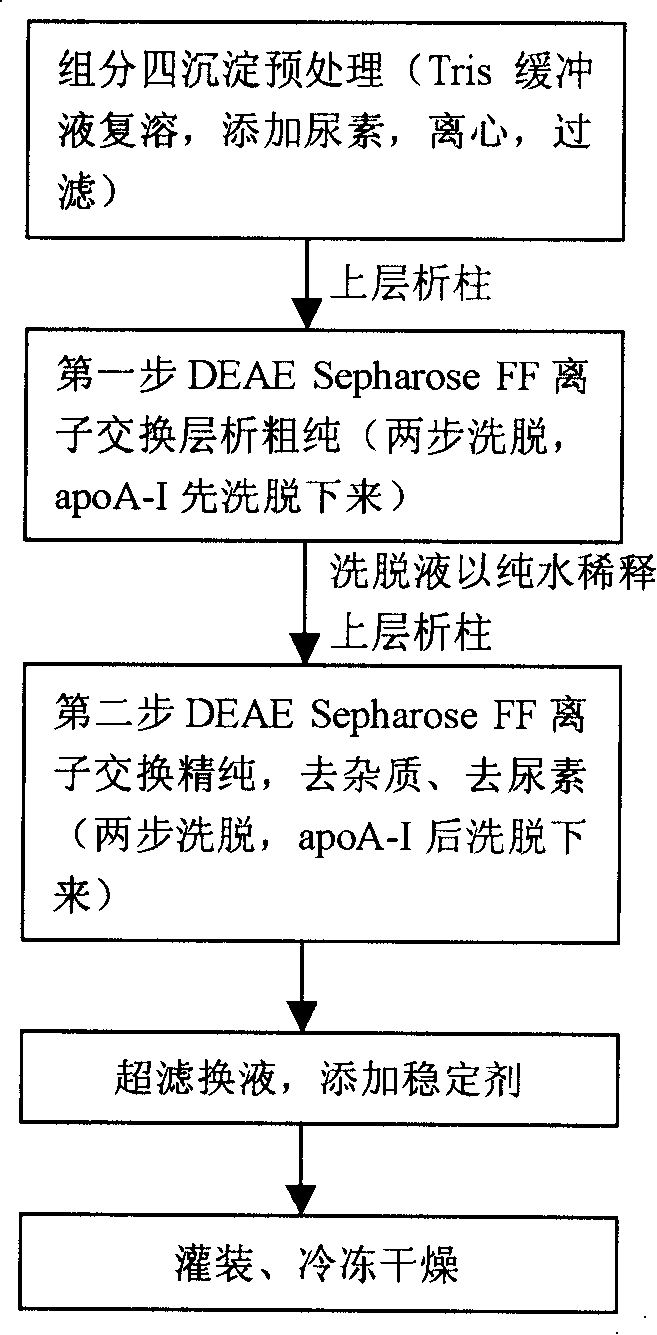
[0049] For the method for preparing apoA-I provided by the invention, refer to the attached figure 1 A flow chart, which includes the following steps:
[0050] (1) Component four precipitation pretreatment
[0051]The fourth component is the human plasma obtained by the cohn low-temperature ethanol method (Cohn E J, StrongLE, Huges WL, et al. Preparation and properties of serum and plasma proteins IVA system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. Amer Chem Soc, 1946, 68: 459-475.). Dissolve the precipitate of component four in the buffer solution, then add a certain concentration of urea, and mix thoroughly to denature the proteins such as apoA-I in component four. The so-called denaturation is reversible.
[0052] The concentration of the added urea is 1-8M, preferably 3-7M, more preferably 5-6M. The weight ratio of component four precipitation to the added urea is 1:30-300, preferably 1:90-240, more pr...
Embodiment 1
[0085] Preparation of apoA-I①
[0086] 0.2 g of component four, the chromatographic column adopts 5ml DEAE anion exchange prepacked column (purchased from GE Healthcare company), the purification equipment is AKTA EXPLORER 100 (purchased from GE Healthcare company), and the conductometric measurement adopts the conductivity detection device that AKTA EXPLORER 100 comes with .
[0087] (1) Component four precipitation pretreatment
[0088] 0.2 g of component four precipitate was dissolved in 100 ml of Tris buffer solution with pH 7.8 at 4°C, and urea was added to a final concentration of 6 mol / L, and mixed well. Centrifuge at 8000 rpm to remove insoluble impurities, and then filter with a 0.45 μm membrane filter.
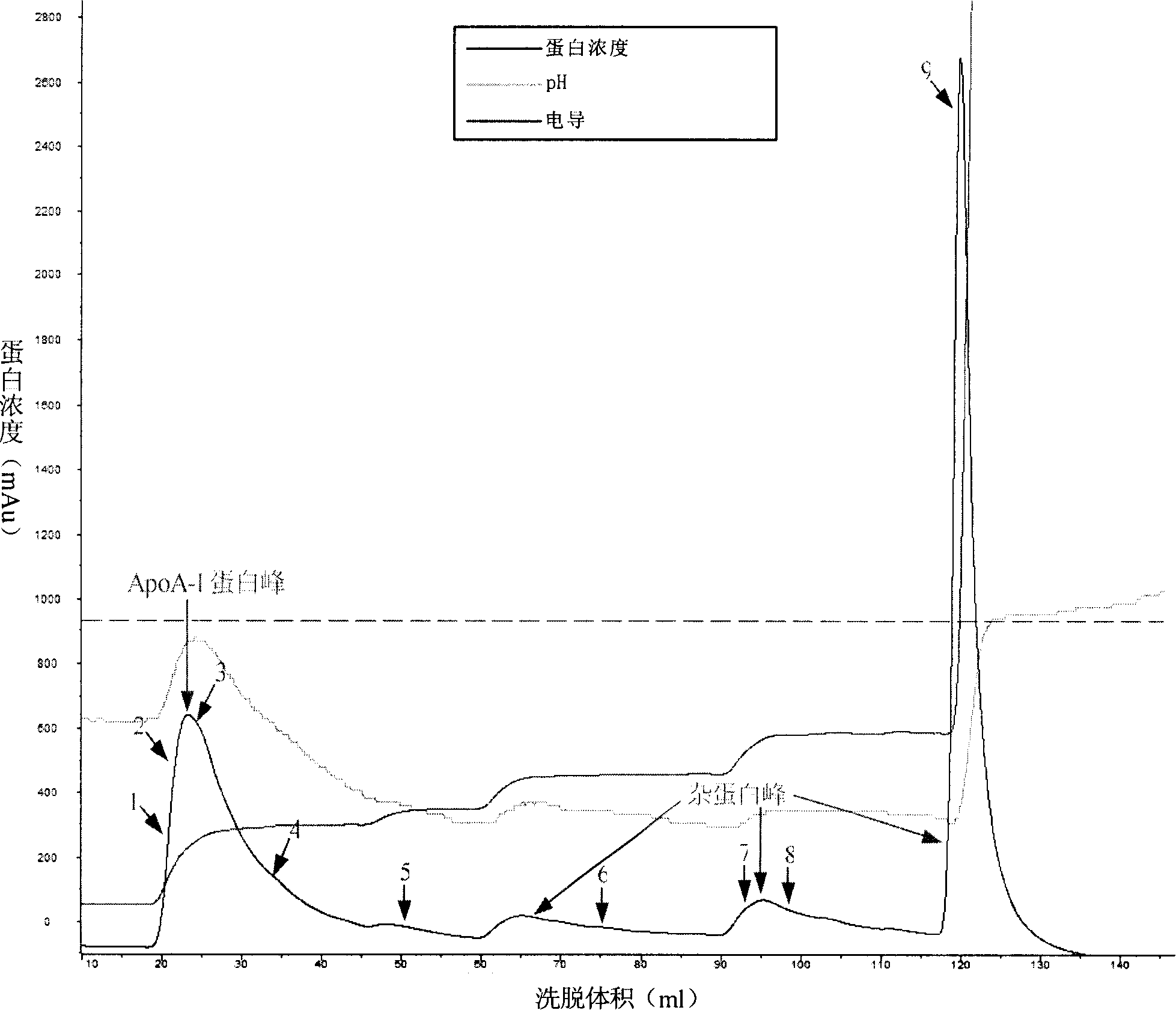
[0089] (2) The first DEAE anion exchange column chromatography
[0090] The DEAE column is balanced with Tris buffer solution (pH7.8) containing 6mol / L urea, and the filtrate of step (1) flows through the DEAE anion exchange chromatography column at a flow rate of...
Embodiment 2
[0098] Preparation of apoA-I②
[0099] Take by weighing 0.2 grams of component four, and the experimental equipment is the same as in Example 1.
[0100] (1) Component four precipitation pretreatment
[0101] 0.2 g of the precipitate of component four was dissolved in 100 ml of Tris buffer at pH 7.8 at 4°C, and urea was added to a final concentration of 1 mol / L, and thoroughly mixed. Centrifuge at 8000 rpm to remove insoluble impurities, and then filter with a 0.45 μm membrane filter.
[0102] (2) The first DEAE anion exchange column chromatography
[0103] The DEAE column was balanced with Tris buffer (pH7.8) containing 1mol / L urea, and the filtrate from step (1) flowed through the DEAE anion-exchange chromatography column at a flow rate of 1ml / min, and apoA-I and miscellaneous proteins were bound to the column.
[0104] Elute apoA-I with Tris buffer containing 1mol / L urea (conductivity 3.5ms / cm, pH7.8), the eluate volume is about 20ml; then wash with Tris buffer pH 7.8 co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com