Method for producing monoammonium phosphate by metathesis method
A technology of monoammonium phosphate and metathesis, which is applied in the direction of phosphate, phosphorus oxyacid, etc., can solve the problems of loss of phosphorus element, increase of production cost of monoammonium phosphate, and influence of total phosphorus yield, etc., so as to reduce production cost and improve production cost. The effect of resource utilization and good social effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
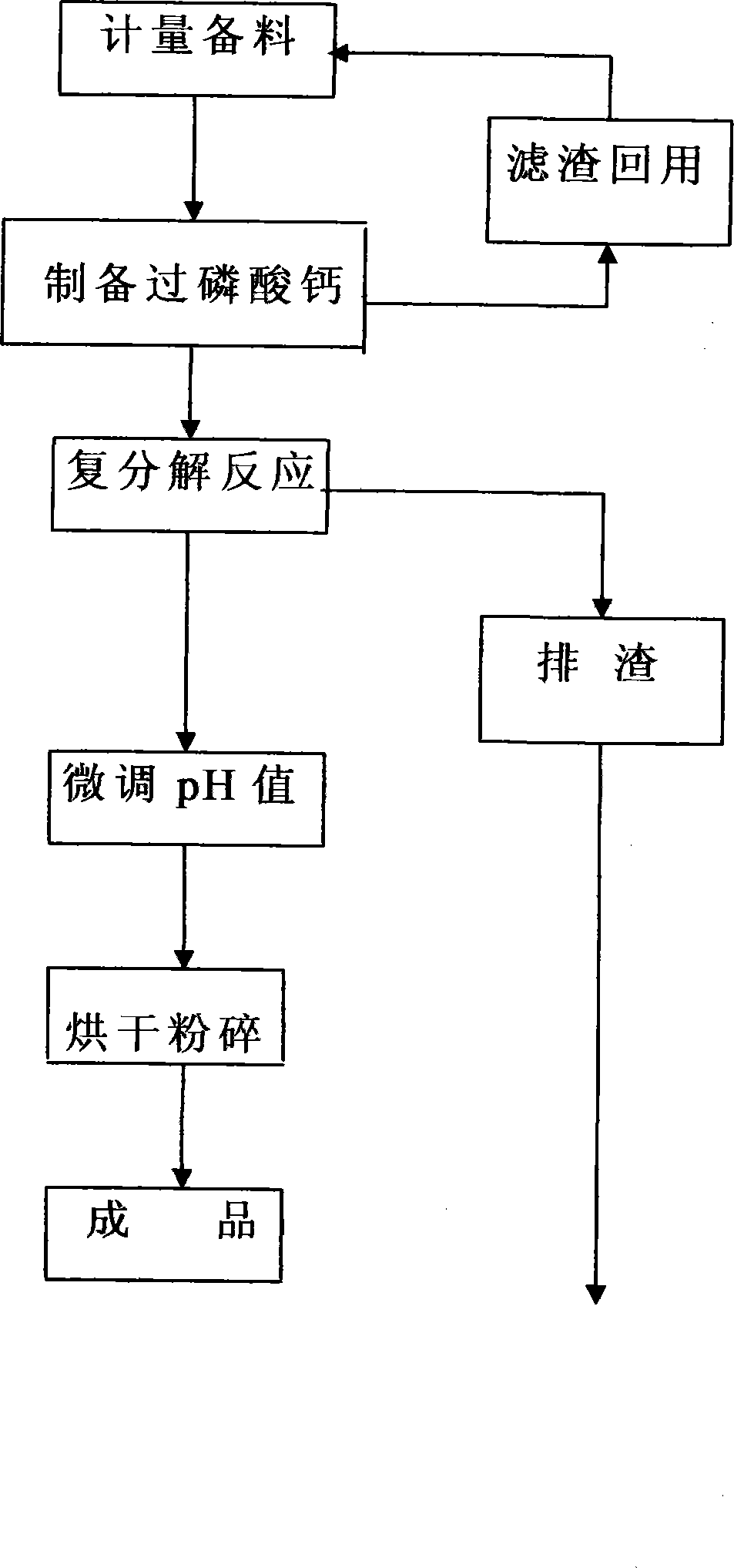
[0032] The method that the present invention proposes produces monoammonium phosphate with metathesis, and it carries out by the step of following order successively:
[0033] (1) Measuring and preparing raw materials: take raw materials by weighing the following parts by weight:
[0034] Contains P 2 o 5 1 part of 30% phosphate rock powder,
[0035] Contains H 2 SO 4 0.5 parts of 95% sulfuric acid,
[0036] Contains (NH 4 ) 2 SO 4 0.35 parts of 99% ammonium sulfate.
[0037] (2) Preparation of superphosphate:
[0038] First, take 60% phosphate rock powder raw material by weight and put it into the reaction tank equipped with agitator and steam heating device, add water according to 4 times the weight of the phosphate rock powder raw material used, and gradually add sulfuric acid under the working condition of dilution and stirring , heating with steam, controlling the reaction temperature to 80°C, under the condition of continuous and uniform stirring, carry out ...
Embodiment 2
[0050] The method that the present invention proposes produces monoammonium phosphate with metathesis, and it carries out by the step of following order:
[0051] (1) Measuring and preparing raw materials: take raw materials by weighing the following parts by weight:
[0052] Contains P 2 o 5 1 part of 25% phosphate rock powder,
[0053] Contains H 2 SO 4 0.4 parts of 95% sulfuric acid,
[0054] Contains (NH 4 ) 2 SO 4 0.3 parts of 99% ammonium sulfate.
[0055] (2) Preparation of superphosphate:
[0056] First, take 50% phosphate rock powder raw material by weight and put it into the reaction tank equipped with agitator and steam heating device, add water according to 3.5 times the weight of the phosphate rock powder raw material used, and gradually add sulfuric acid under the working condition of dilution and stirring , heating with steam, controlling the reaction temperature to 75°C, under the condition of continuous and uniform stirring, carry out the followin...
Embodiment 3
[0068] It proceeds through the steps in the following order:
[0069] (1) Measuring and preparing raw materials: take raw materials by weighing the following parts by weight:
[0070] Contains P 2 o 5 1 part of 20% phosphate rock powder,
[0071] Contains H 2 SO 4 0.3 parts of 95% sulfuric acid,
[0072] Contains (NH 4 ) 2 SO 4 0.22 parts of 99% ammonium sulfate.
[0073] (2) Preparation of superphosphate:
[0074] First, take 55% phosphate rock powder raw material by weight and put it into the reaction tank equipped with agitator and steam heating device, add water according to 3 times the weight of the phosphate rock powder raw material used, and gradually add sulfuric acid under the working condition of dilution and stirring , heating with steam, controlling the reaction temperature to 85°C, under the condition of continuous and uniform stirring, carry out the following chemical reaction for 6 hours:
[0075] Ca 5 F(PO 4 ) 3 +5H 2 SO 4 -3H 3 PO 4 +5CaSO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com