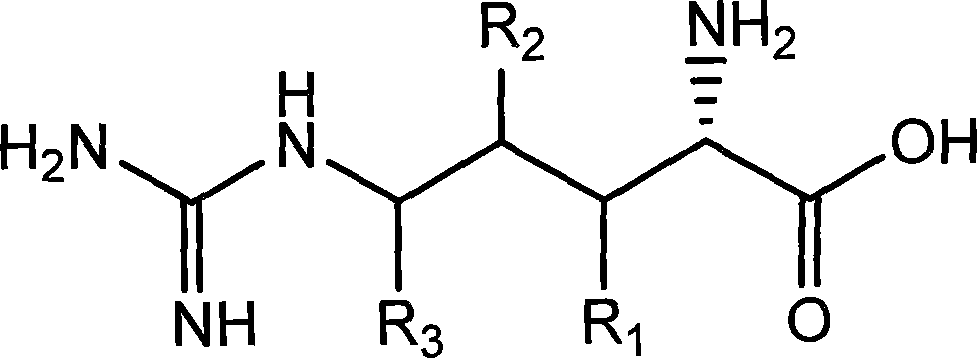
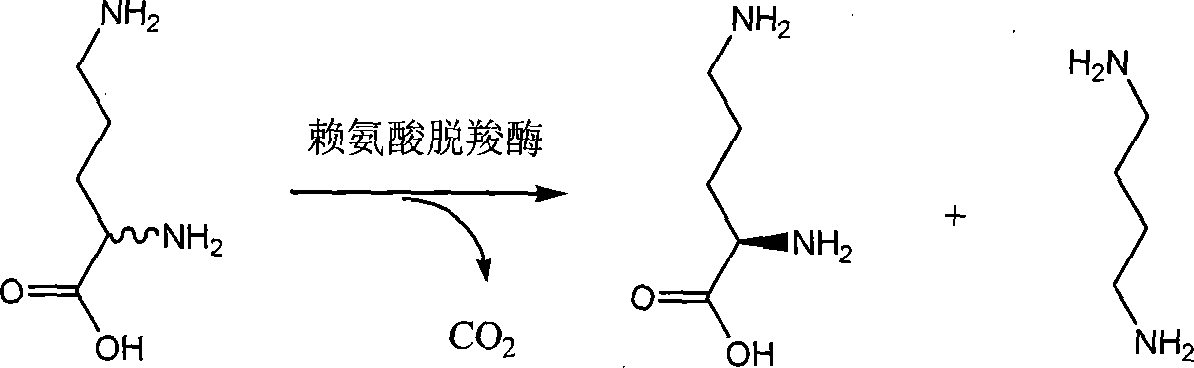
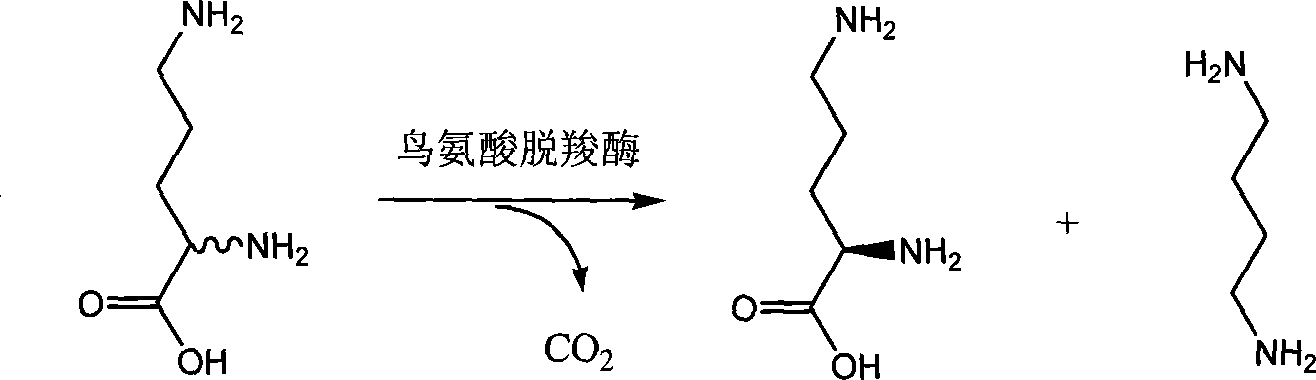
Chiral method for preparing D-ornithine and putrescine or derivatives thereof
A derivative, ornithine technology, applied in the field of biotransformation and separation of chiral compounds, can solve the problems of inconvenient to obtain polyamine compounds, inability to prepare D-ornithine, environmental pollution, etc., and achieves short conversion time and low price. , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1. Put the Hafnia alvei AS 1.1009 bacterial strain in the following 1000mL culture medium: 2% sucrose, 1% peptone, 2% corn steep liquor, 0.1% yeast extract, (NH 4 ) 2 SO 4 0.1%, L-Lysine 1%. The fermentation conditions are as follows: temperature 35°C, inoculum size 6%, medium loading volume 100mL, fermentation time 14h. Wet cells were obtained by centrifugation at 4000rpm for 15min.
[0044] 2. Add cells to 600mL transformation solution containing 10% DL-ornithine obtained by racemization reaction, add 6mL 0.5% Tween-80 and 600mL pH8.0 phosphate buffer, and react at 37°C 24h. .
[0045] 3. Concentrate the conversion solution to 200 mL, add 2 g of activated carbon for decolorization. Continue to concentrate the decolorized solution to 100mL, add 200mL of 95% ethanol and stir evenly, cool and crystallize, filter and dry in vacuum to obtain a dry weight of 37.5g of crystals, [α] D 20 =-11.5 (C=5.5, H 2 (0), the gained mother liquor is adsorbed by JK008 cationic ...
Embodiment 2
[0047] 1. Select the Hafnia alvei AS 1.1009 bacterial strain, the seed culture and fermentation conditions are the same as in Example 1, and wet cells are obtained.
[0048] 2. Add the cells to 600mL transformation solution containing 10% DL-ornithine obtained by racemization reaction, add 6mL 0.1% cetyltrimethylammonium bromide and 600mL pH7.0 diphosphate Sodium hydrogen-citric acid buffer solution, react at 30°C for 36h.
[0049] 3. Concentrate the conversion solution to 200 mL, add 2 g of activated carbon for decolorization. Continue to concentrate the decolorized solution to 100mL, add 200mL of 95% ethanol and stir evenly, cool and crystallize, filter and dry in vacuo to obtain 30.1g of crystal dry weight, [α] D 20 =-10.8 (C=5.5, H 2 (0), the obtained mother liquor is adsorbed by JK008 cationic resin, eluted with 3% ammonia water, and the eluent is decolorized by activated carbon and then concentrated, and the concentrated solution is adjusted to pH 10 to obtain putresc...
Embodiment 3
[0051] 1. Put the Hafnia alvei AS 1.1009 strain in the following 1000mL medium: 2% glucose, 1% soybean cake hydrolyzate, 2% corn steep liquor, 0.1% yeast extract, (NH 4 ) 2 SO 4 0.1%, L-ornithine 0.5%. The fermentation conditions are as follows: a temperature of 35° C., an inoculum size of 5%, a culture medium loading capacity of 100 mL, and a fermentation time of 20 h. Wet cells were obtained by centrifugation at 4000rpm for 15min.
[0052] 2. Add the cells to 600mL of transformation solution containing 10% DL-ornithine obtained by racemization reaction, add 6mL of 0.5% Tween-80 and 600mL of pH9.0 borax-hydrochloric acid buffer solution, 40°C Reaction 48h.
[0053] 3. Concentrate the conversion solution to 200 mL, add 2 g of activated carbon for decolorization. Continue to concentrate the decolorized solution to 100mL, add 200mL of 95% ethanol and stir evenly, cool and crystallize, filter and vacuum dry to obtain a crystal dry weight of 35.5g, [α] D 20 =-11.2 (C=5.5, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com