Synthesis method and application of 5,7,4'-trihydroxy-3',5'-dimethoxyflavone
A technology of dimethoxyflavone and synthesis method, which is applied in the field of synthesis and application of 5,7,4'-trihydroxy-3',5'-dimethoxyflavone, and can solve the problem of increasing production cost and pesticide residue Health problems, environment, unfriendliness and other problems, to prevent spore germination, promote sustainable development, and increase rice yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
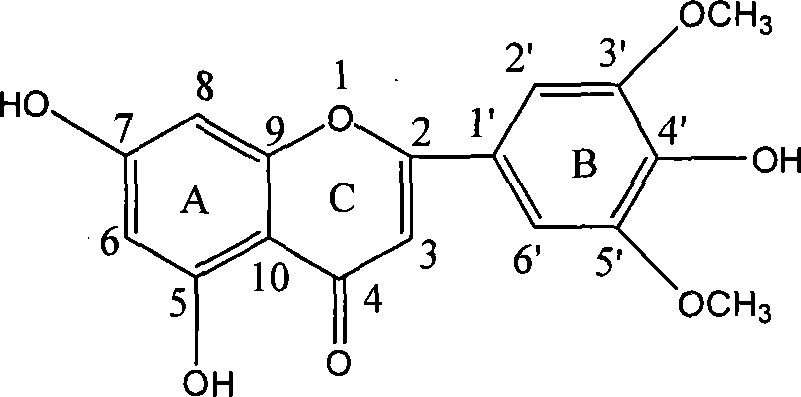
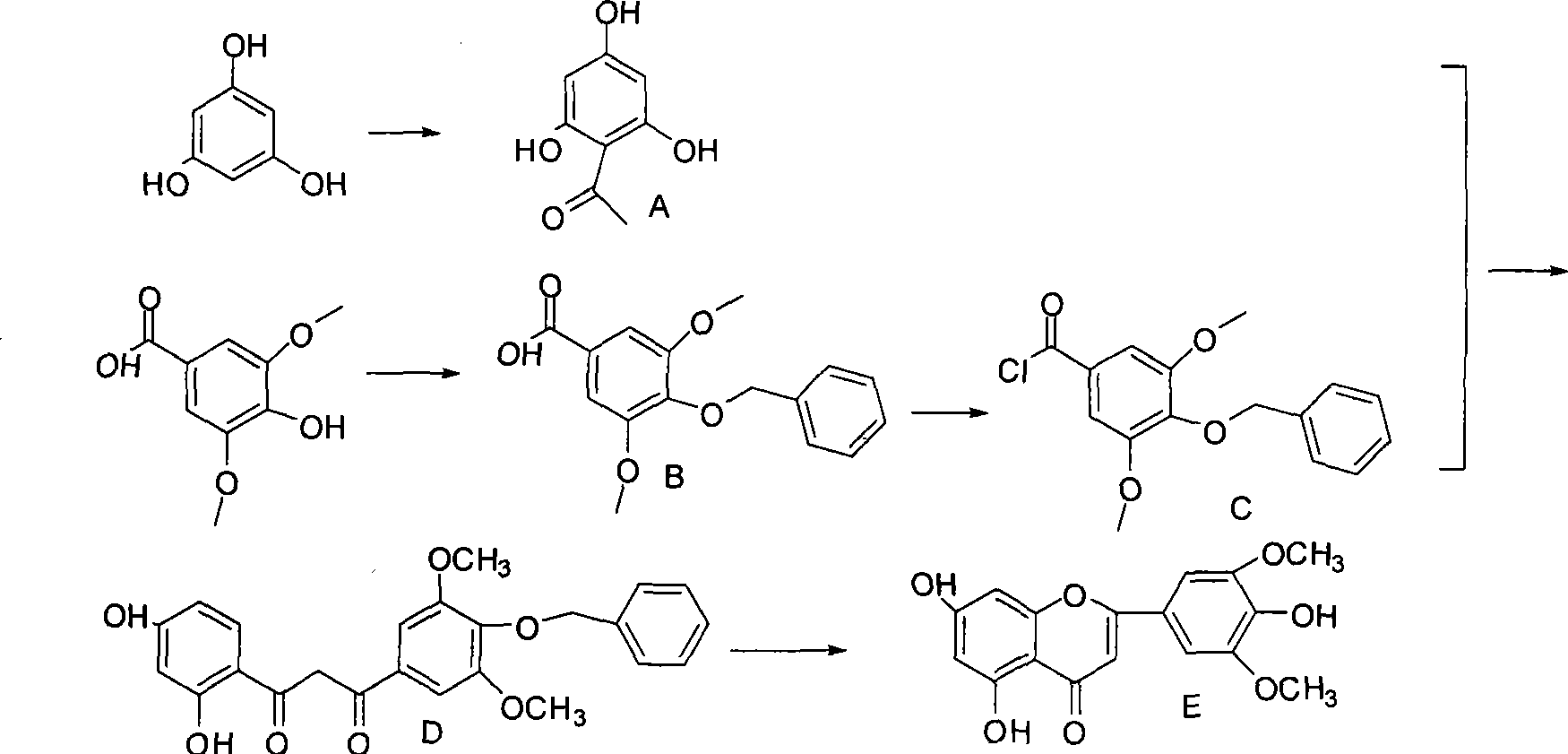
[0024] Example 1 Synthesis of 5,7,4'-trihydroxy-3',5'-dimethoxyflavone
[0025]
[0026] 1) Synthesis of compound A (Hoesch reaction)
[0027] 5.0g of anhydrous phloroglucinol and 3.5mL of acetonitrile dissolved in 165mL of dry ether, cooled to -10°C in an ice-salt bath, added 2.7g of molten zinc chloride under stirring, and then introduced dry hydrogen chloride gas for 2h, and then reacted The bottle was sealed in the refrigerator and placed for 1d. The next day, hydrogen chloride gas was continued for 2h, and then placed in the refrigerator for 1d. An orange solid precipitated out. Filtered. The filter cake was washed twice with anhydrous ether, then dissolved in 250mL of water and transferred to a round bottom. The flask was heated to reflux for 1 hour and left to stand overnight. A yellow solid was precipitated and recrystallized twice with water to obtain 5.8 g of light yellow needle-like crystals with a yield of 87%. 1 H NMR (300MHz, DMSO) δ 2.37 (s, 3H, CH 3 C=O), 7.40 (s...
Embodiment 2
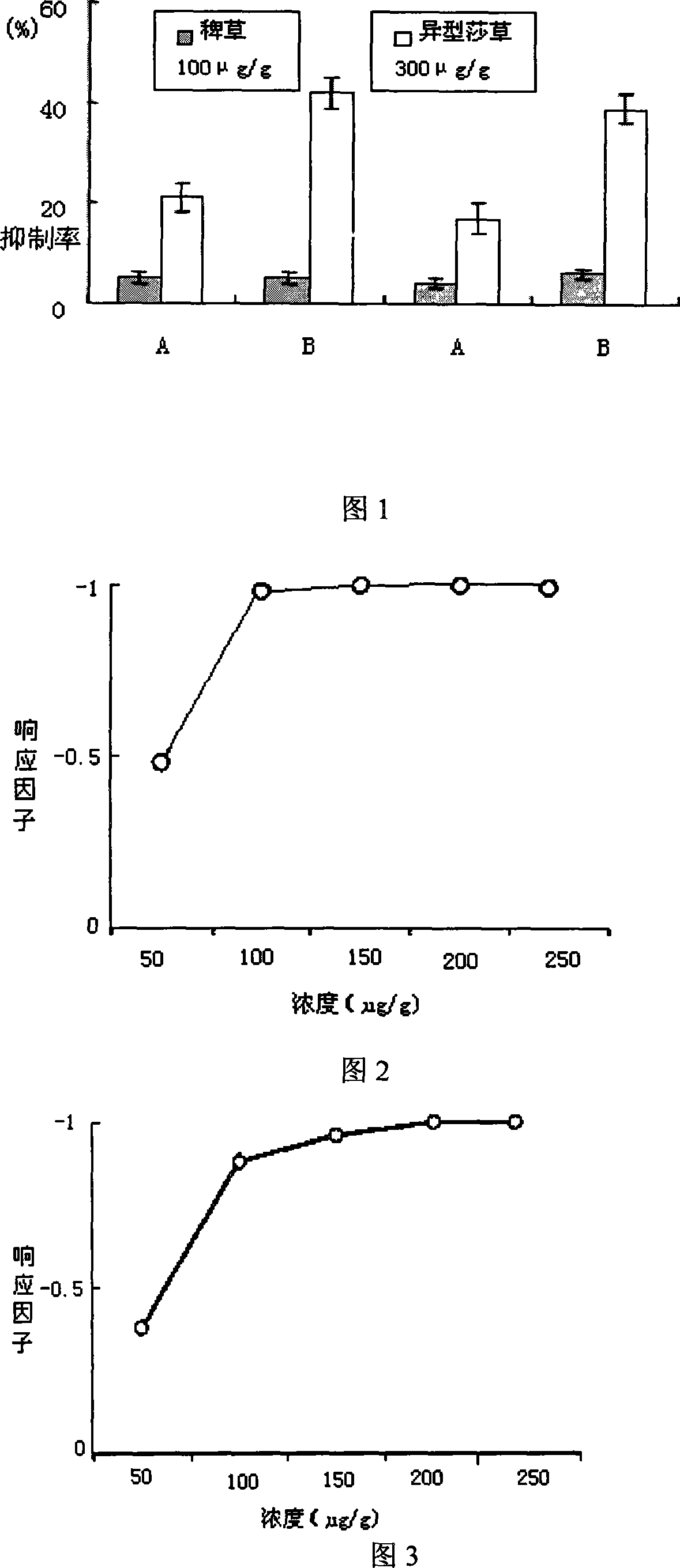
[0037] 1) Petri dish anti-grass biological activity test
[0038] Put a layer of filter paper on the bottom of a petri dish with a diameter of 9 cm, and then add an aqueous solution of flavonoids with a set concentration, and set a petri dish with only distilled water as a control. The pre-germinated seeds of barnyardgrass or heterotypic sedge were sown evenly into a petri dish with 50 seeds per dish, and the petri dish was placed in an incubator and grown for 7 days under the conditions of 25±1°C and 12h light. Harvest the seedlings in each petri dish at 80°C. Weigh the dry weight after drying for 12 hours.
[0039] 2) Potted plant anti-grass biological activity test
[0040] 50 barnyardgrass or heteromorphic sedge seeds were evenly sown into a pot (5cm×7cm) containing 150g paddy soil. After the seedlings emerged, each pot was thinned to 10 plants, and a set concentration of flavonoid aqueous solution was applied to the pot. Only add distilled water. The pot is placed in an incub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com