Method for rapidly removing metal copper ion from waste water
A technology for metal copper and copper ions, which is applied in the field of rapid removal of metal copper ions in wastewater, can solve the problems of high energy consumption, cumbersome operation and low removal rate, and achieves the effects of high efficiency, less time-consuming and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The activation of the nylon membrane and the modification of the bonded chitosan, the specific steps are as follows:
[0028] (1) The nylon membrane was first hydrolyzed in HCl with a molar concentration of 1mol / L at 25°C for 24h, and then activated with formaldehyde. Ten hydrolyzed nylon membranes were immersed in 20ml of formaldehyde solution (>36.5wt.%), added with 0.2ml of phosphoric acid (85wt.%), reacted at 60°C for 7h, and washed with hot water at 40-50°C for several times.
[0029] (2) The above-mentioned formaldehyde-activated nylon membrane is immersed in 10ml mass fraction of 1.5% chitosan solution (dissolved with 1vol.% acetic acid), reacted at room temperature for 1h, then transferred to an 80°C oven, took it out after 1h, and used 1vol .% acetic acid and deionized water to wash away unreacted chitosan. The content of chitosan on the nylon film can be tested according to the ninhydrin method.
Embodiment 2
[0031] Bond coupling agent is carried out the activation of chitosan membrane, concrete steps are as follows:
[0032] (1) 10 nylon membranes after the above modification are immersed in 4.6ml epichlorohydrin, 46ml molar concentration of 2mol / L sodium hydroxide and 0.017g sodium borohydride mixed solution, react at room temperature for 2h, and then directly mix Add 23ml of oxochloropropane and 46ml of sodium hydroxide with a molar concentration of 2mol / L to the liquid, react at 60°C for 12h, and wash with deionized water several times.
[0033] (2) Immerse the above-mentioned cyclopropane-bound membrane into a mixed solution containing 86 mmol of iminodiacetic acid (IDA), 0.14 g of sodium borohydride and 114.4 ml of sodium carbonate with a molar concentration of 2 mol / L, and react with constant temperature oscillation at 60 ° C for 12 h, Wash with deionized water, 5vol.% acetic acid and deionized water several times.
Embodiment 3
[0035] Use the modified nylon affinity membrane to absorb copper ions and optimize the conditions. The specific steps are as follows:
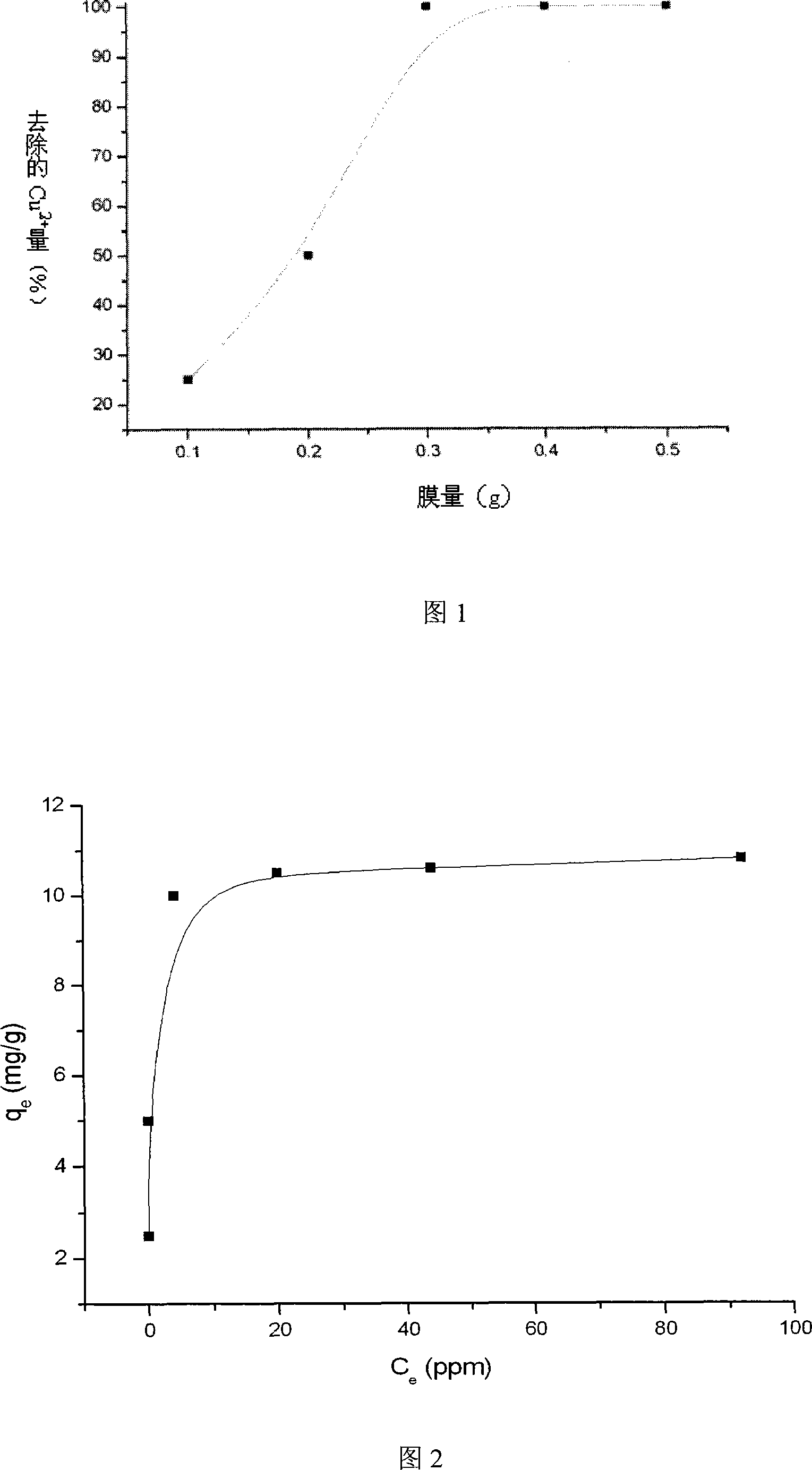
[0036] (1) Determination of the amount of reaction film
[0037] Stack 1, 2, 3, 5, and 10 sheets of modified nylon membranes to form a membrane stack, put them into the membrane bridge, and send 30ml of 100ppm copper sulfate solution with a peristaltic pump, and circulate for 3 hours at a flow rate of 2.0ml / min. The spectrophotometer detects the amount of copper ion adsorption at a wavelength of 740nm. The experimental results are shown in Fig. 1, and the optimum film quantity is 3 films (ie 0.3g film).
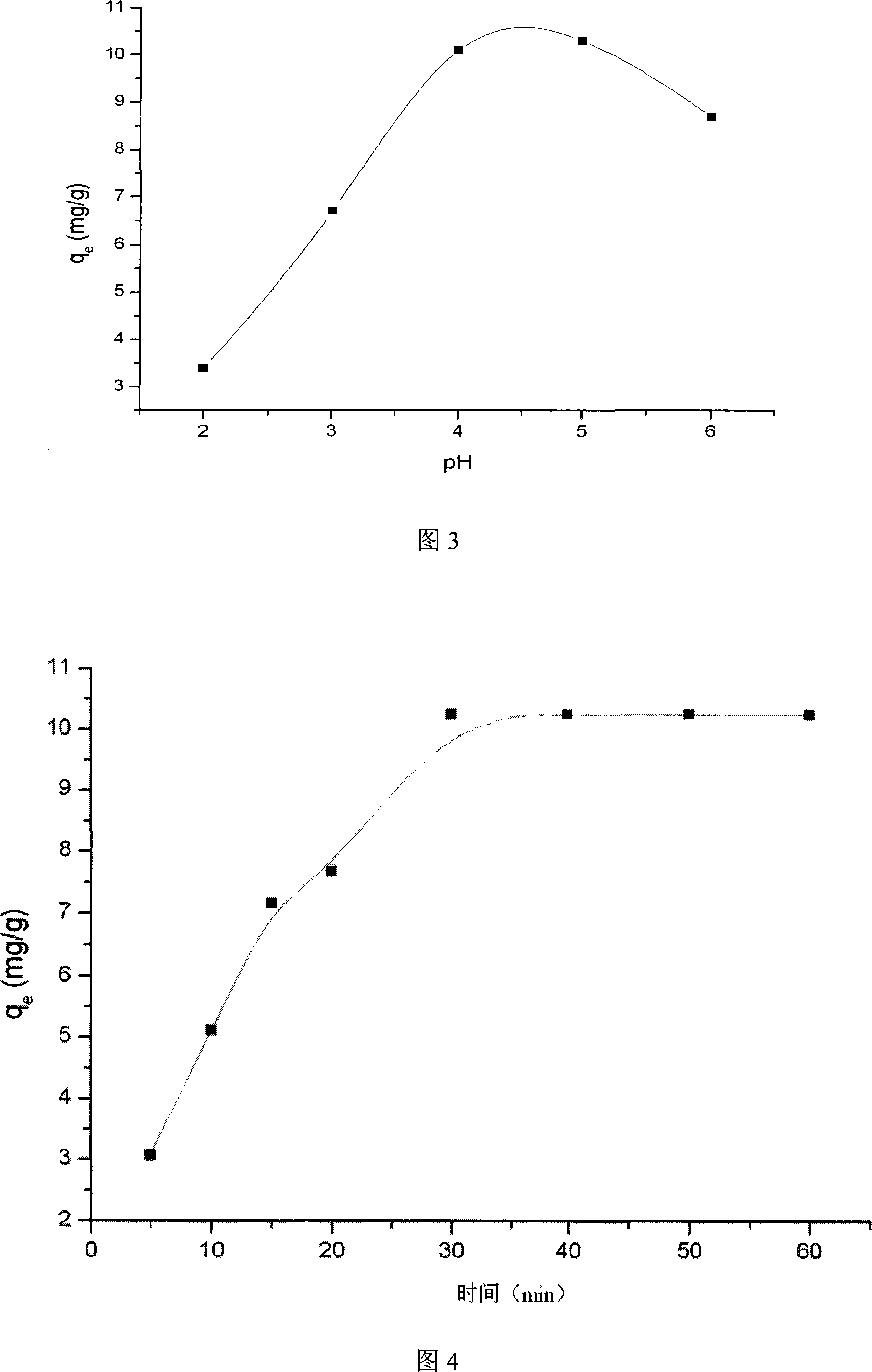
[0038] (2) Determination of reaction initial copper sulfate solution concentration
[0039] Stack 3 modified nylon membranes to form a membrane stack, put them into the membrane bridge, and use a peristaltic pump to send in copper sulfate reaction solution with a concentration ranging from 30ml 25ppm to 200ppm, and circulate the reaction fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com