Bioactive aquagel-conductive polymer nanometer composite material and synthetic material thereof
A nano-composite material, conductive polymer technology, applied in the direction of conductive materials dispersed in non-conductive inorganic materials, etc., can solve the problems of difficult processing, unfavorable acquisition of nutrients, poor mechanical properties, etc., to achieve short curing time, geometric The effect of easy shape control and low reaction heat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Synthesis of ethylene glycol-lactic acid block copolymer gel and polyaniline nanocomposite:
[0027] (1) Synthesis of ethylene glycol-lactic acid block copolymer:
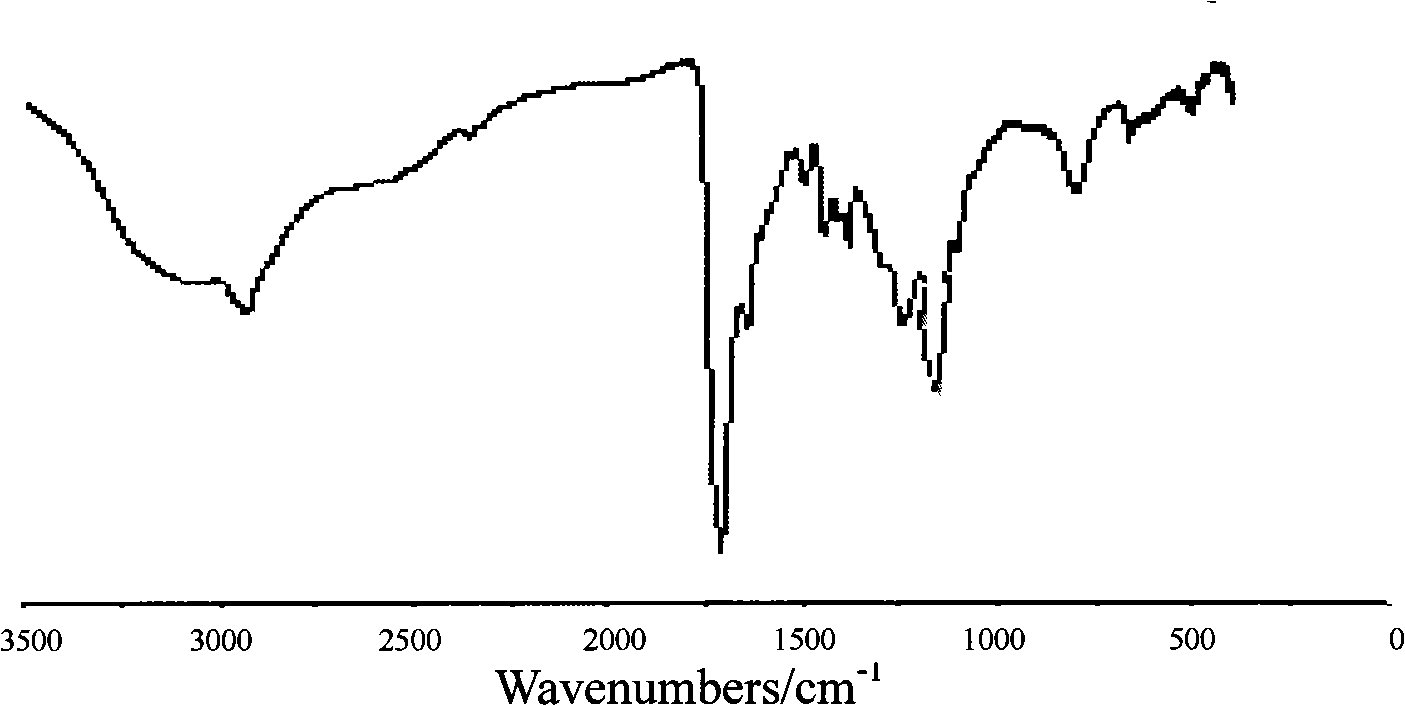
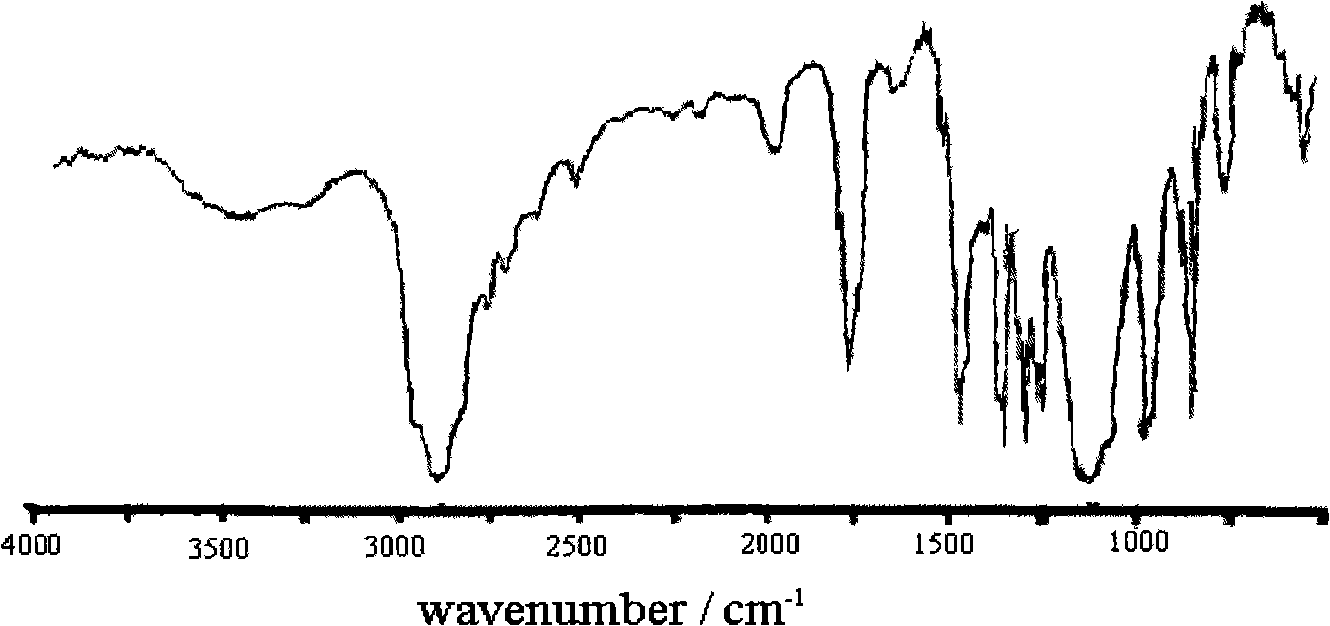
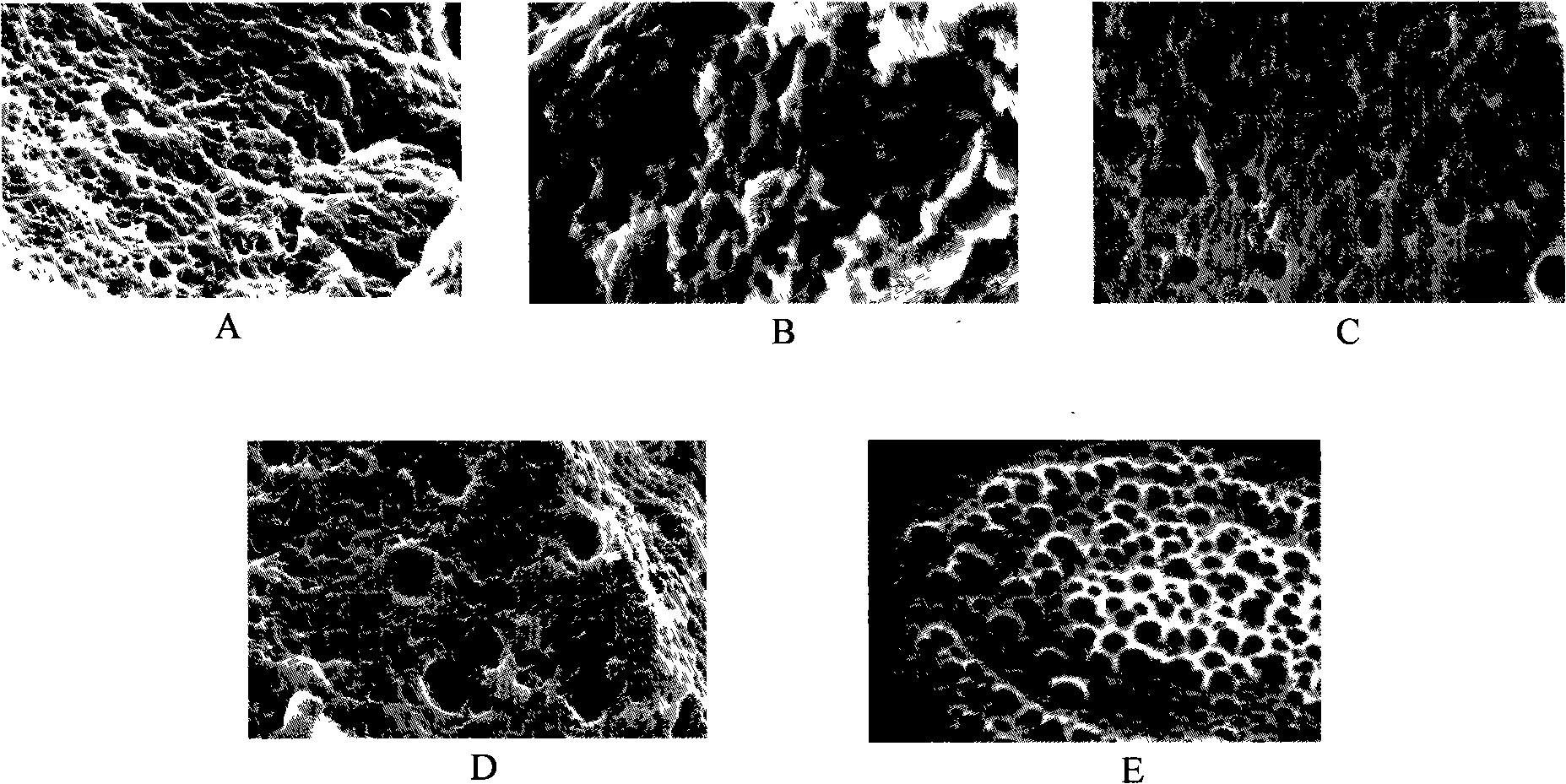
[0028] Under the protection of nitrogen, take 10g PEG (number average molecular weight 200) and 36gdl-lactic acid, and add 23mg of tin octoate into a 100ml three-neck round bottom flask. The reaction mixture was stirred under vacuum at 200°C for 4h, then lowered to 160°C, continued to stir for 2h, and finally cooled to room temperature to obtain an ethylene glycol-lactic acid block copolymer, which was dissolved in dichloroethane and used without Precipitate with water and ether, filter and dry to obtain a purified ethylene glycol-lactic acid block copolymer. From image 3 As can be seen in the SEM photo of A, the synthesized hydrogel membrane material exhibits a honeycomb shape, and these penetrating holes provide a composite space for the conductive polymer.
[0029] (2) Synthesis of ethyl...
Embodiment 2
[0034] Embodiment 2: Synthesis of ethylene glycol-glycolide block copolymer gel and polypyrrole nanocomposite material:
[0035] (1) Synthesis of ethylene glycol-glycolide block copolymer:
[0036] Under nitrogen protection, take 20g PEG (number average molecular weight 400) and 22.8g glycolide (0.2mol, M144), and add 25mg tin octoate into a 100ml three-neck round bottom flask. The reaction mixture was stirred in vacuum at 190°C for 5h, then lowered to 150°C, stirred for 4h, and finally cooled to room temperature. The ethylene glycol-glycolide block copolymer is obtained, and the product is dissolved in dichloroethane, precipitated with anhydrous ether, filtered, and dried to obtain a purified ethylene glycol-glycolide block copolymer. From image 3 As can be seen in the SEM photo of B, the synthesized hydrogel membrane material exhibits a honeycomb shape, and these penetrating holes provide a composite space for the conductive polymer.
[0037] (2) Synthesis of ethylene gl...
Embodiment 3
[0043] Embodiment 3: Synthesis of ethylene glycol-ε-caprolactone block copolymer gel and polyaniline nanocomposite material:
[0044] (1) Synthesis of ethylene glycol-ε-caprolactone block copolymer:
[0045] Under nitrogen protection, take 30g PEG (number average molecular weight 600) and 45.6gε-caprolactone (0.4mol, M114), and add 48mg tin octoate into a 100ml three-neck round bottom flask. The reaction mixture was stirred under vacuum at 210°C for 3h, then lowered to 170°C, continued to stir for 1.5h, and finally cooled to room temperature. To obtain ethylene glycol-ε-caprolactone block copolymer, dissolve the product in dichloroethane, precipitate with anhydrous ether, filter, and dry to obtain purified ethylene glycol-ε-caprolactone block copolymer . From image 3 It can be seen in the SEM photo of C that the synthesized hydrogel membrane material exhibits a honeycomb shape, and these penetrating holes provide a composite space for the conductive polymer.
[0046] (2) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com