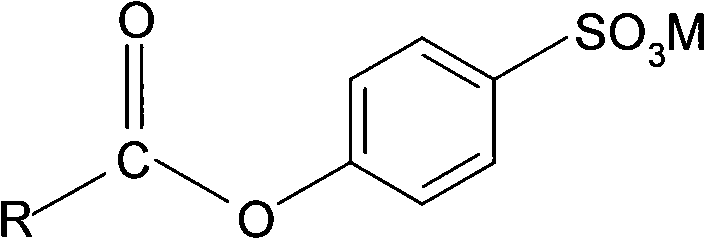
Environment-friendly type preparing method for H2O2 low-temperature bleaching assistant
A kind of H2O2, environment-friendly technology, applied in the direction of sulfonic acid preparation, organic chemistry, etc., to achieve the effect of simple and easy to obtain raw materials, simple operation and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9
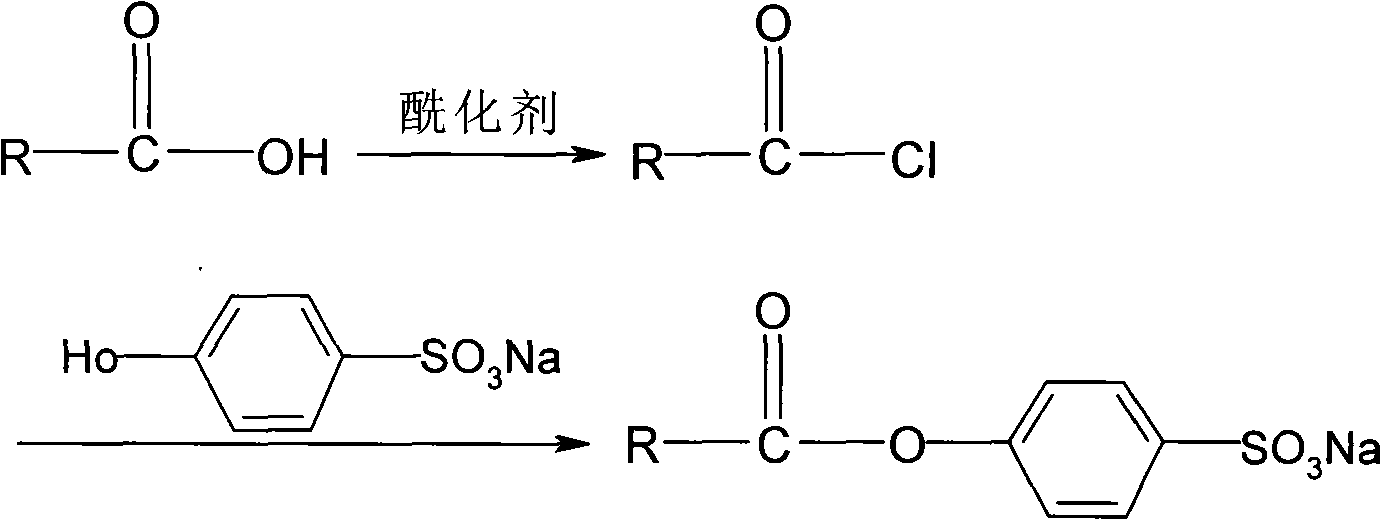
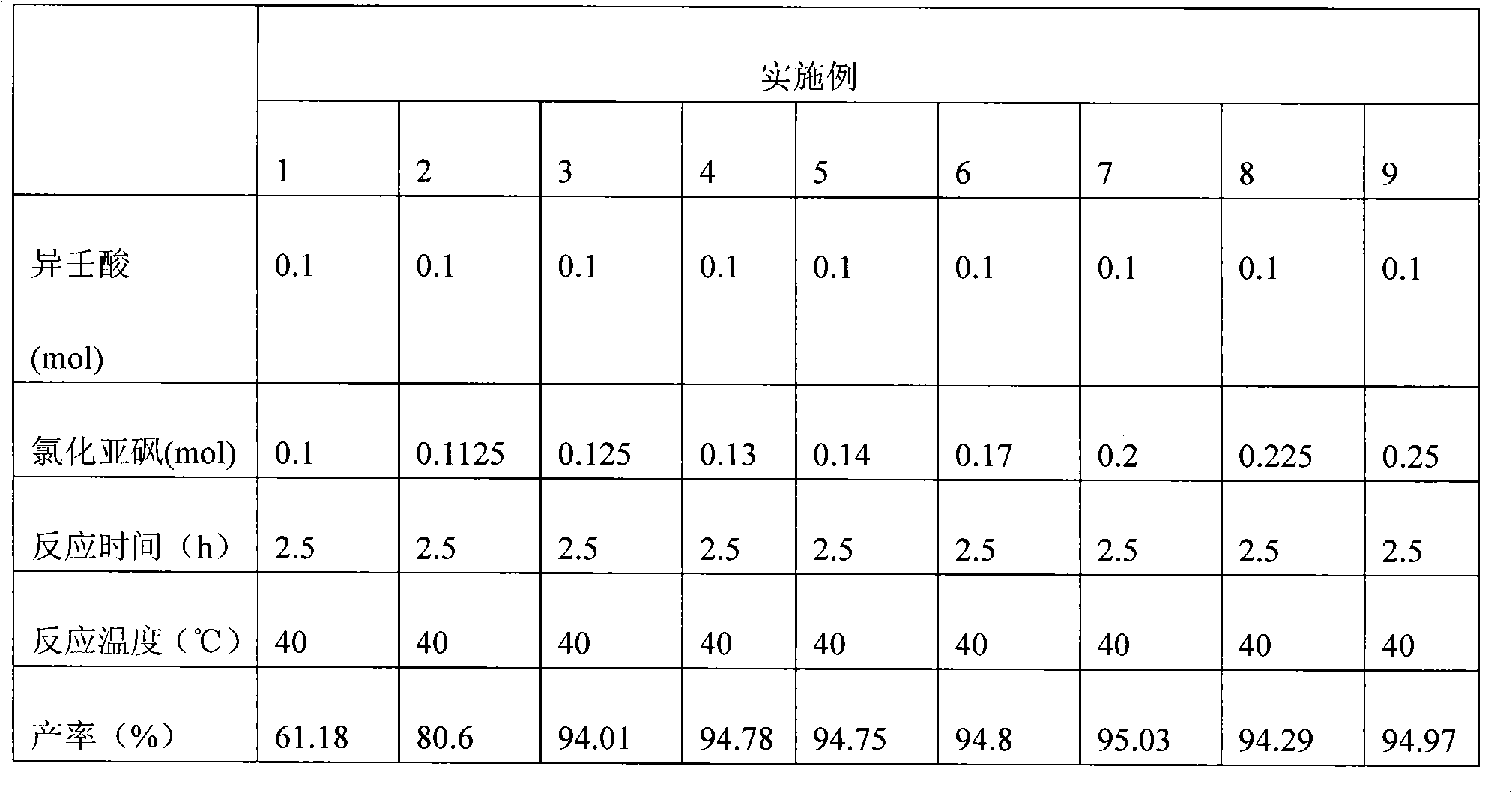
[0027] Add 15.82g (0.1mol) isononanoic acid in the there-necked flask that reflux condenser, constant pressure dropping funnel and thermometer are housed, start to stir, slowly drip the sulfur oxychloride ( See Table 1 for details), control the rate of addition to complete the dropwise addition in about 1 hour; allow it to react for 30 minutes, then raise the temperature to 40°C for reflux reaction for 2.5 hours. Then carry out normal pressure distillation to remove excess thionyl chloride, and vacuum distillation collects the product isononanoyl chloride. The test results are shown in Table 1.
[0028] Table I
[0029]
[0030] 2. The influence of reaction temperature on the synthesis of isononanoyl chloride
Embodiment 10~15
[0032] Add 15.82g (0.1mol) isononanoic acid to a three-necked flask equipped with a reflux condenser, a constant pressure dropping funnel and a thermometer, start stirring, and slowly add 15.47g (0.13mol) thionyl chloride dropwise at room temperature to control the rate of addition The dropwise addition was completed in about 1 hour; after allowing it to react for 30 minutes, the temperature was raised to the temperature shown in Table 2 for reflux reaction for 2.5 hours. Then carry out normal pressure distillation to remove excess thionyl chloride, and vacuum distillation collects the product isononanoyl chloride. The test results are shown in Table II.
[0033]
[0034] 3. The influence of the amount ratio of raw materials on the synthesis of sodium nonanoyl chloride benzene sulfonate
Embodiment 16~21
[0036] Prepare a certain amount of lye with a pH value of 11 with the mixture of sodium hydroxide, sodium carbonate, and sodium bicarbonate with the same mass fraction and volume ratio of 1:4:1, and 1.96g (0.01mol) of p-hydroxybenzenesulfonic acid Sodium was added wherein, stirred and dissolved, then added 5% phase-transfer catalyst tetrabutylammonium bromide, continued to stir for 5 minutes and then added dropwise nonanoyl chloride (see Table 3) in different molar ratios with sodium p-hydroxybenzenesulfonate, and the reaction Carried out at normal temperature (25°C). After reacting for 40 minutes, the reaction solution was evaporated by rotary evaporation, centrifugally filtered and vacuum-dried to obtain the product sodium isononanoyloxybenzenesulfonate.
[0037] Table three
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com