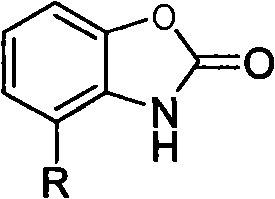
Benzoxazole ketones derivative and preparation method thereof
A technology for benzoxazolones and derivatives, applied in the field of novel compounds and their preparation, can solve problems such as no 4-position substituted derivatives, achieve suitable large-scale industrial production, simple operation, and single reaction results. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
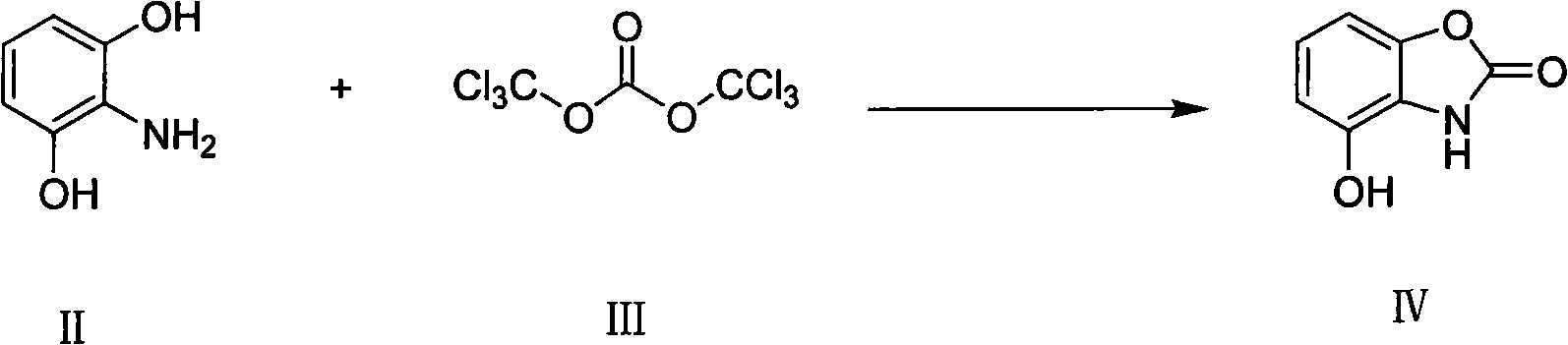
[0030] Example 1: Preparation of 4-hydroxy-benzoxazolone IV.
[0031] 2-Amino-resorcinol was placed in a 250mL round-bottomed flask, and 100mL of tetrahydrofuran solution was added dropwise, then a tetrahydrofuran solution of triphosgene (BTC), and 2-amino-resorcinol and triphosgene were added dropwise. The molar ratio is 1:1.2~2.0, the reaction solution is brown, refluxed for 2~5h under the protection of nitrogen, the reflux temperature is 55~75°C, column chromatography (6%CH 3 OH / CH 2 Cl 2 Elution), a brownish-yellow solid was obtained with a yield of 78.8%. 1 H NMR (600MHz, CD 3 OD): δ6.643(d, J=7.959Hz, 1H, ArH), 6.737(d, J=7.959Hz, 1H, ArH), 6.879(t, J=8.223Hz, 1H, ArH), 10.127(s , 1H, OH), 11.540 (d, 1H, NH). ESI-MS m / z: 150.23 ([M-H] - ).
Embodiment 2
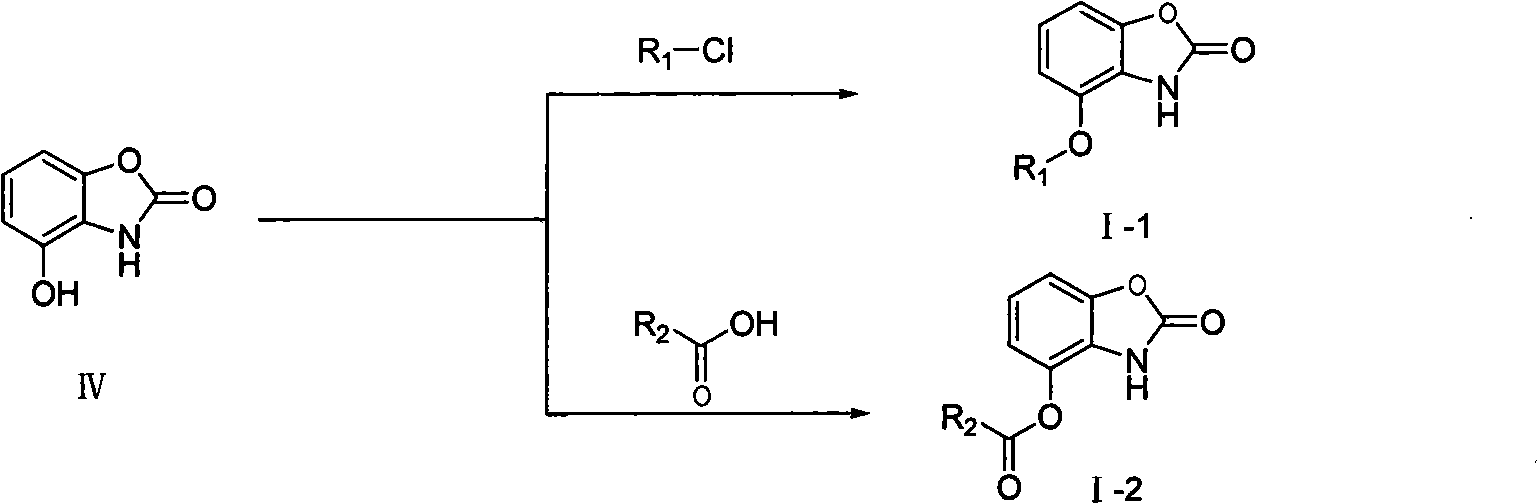
[0032] Example 2: Preparation I-1 of 4-benzoyloxy-benzoxazolone
[0033] Weigh 100mg (0.66mmol) of 4-hydroxy-benzoxazolone IV into a round bottom flask, add anhydrous acetonitrile to dissolve, then add 35mg (0.99mmol) of ammonia water, add dropwise 139mg (0.99mmol) of benzoyl chloride under ice cooling mmol) in acetone solution, continue to react until the white mist disappears, then react at room temperature for 30min, and reflux at 80°C for 6h. The molar ratio of the above-mentioned compound IV to compound benzoyl chloride and phosphonic acid agent is 1: 1.5: 1.5. Separation by silica gel column chromatography (20% ethyl acetate / petroleum ether) gave 47 mg of white solid powder, yield 27.9%, mp 202.2-202.5°C. 1 H NMR (300MHz, CDCl 3 )δ7.160(m, 3H, ArH), 7.590(t, J=7.336Hz, 2H, ArH), 7.727(t, J=7.336Hz, 1H, ArH), 8.262(d, J=7.336Hz, 2H , ArH), 8.50 (s, 1H, NH). ESI-MS m / z: 256 ([M+H] + ).
Embodiment 3
[0034] Example 3: Preparation I-1 of 4-benzenesulfonyloxy-benzoxazolone
[0035] Weigh 100 mg (0.66 mmol) of 4-hydroxy-benzoxazolone and place it in a round-bottomed flask, add anhydrous tetrahydrofuran to dissolve it, then add 48 mg (0.66 mmol) of diethylamine, and add dropwise 117 mg of benzenesulfonyl chloride ( 0.66mmol) of acetone solution, continue to react until the white mist disappears, then react at room temperature for 30min, and reflux at 60°C for 4h. The molar ratio of the above-mentioned compound IV to compound benzoyl chloride and formic acid agent is 1:1.0:1.0. After separation by silica gel column chromatography (20% ethyl acetate / petroleum ether), 79 mg of white solid powder was obtained, with a yield of 41.1%, and mp 181-185°C. 1 H NMR (600MHz, CDCl 3 )δ6.557(d, J=8.597Hz, 1H, ArH), 6.946(t, J=8.309Hz, 1H, ArH), 7.102(d, J=8.309Hz, 1H, ArH), 7.580(t, J =7.743Hz, 2H, ArH), 7.734(t, J=7.527Hz, 1H, ArH), 7.873(d, J=7.554Hz, 2H, ArH), 8.067(s, 1H, NH). ESI-MS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com