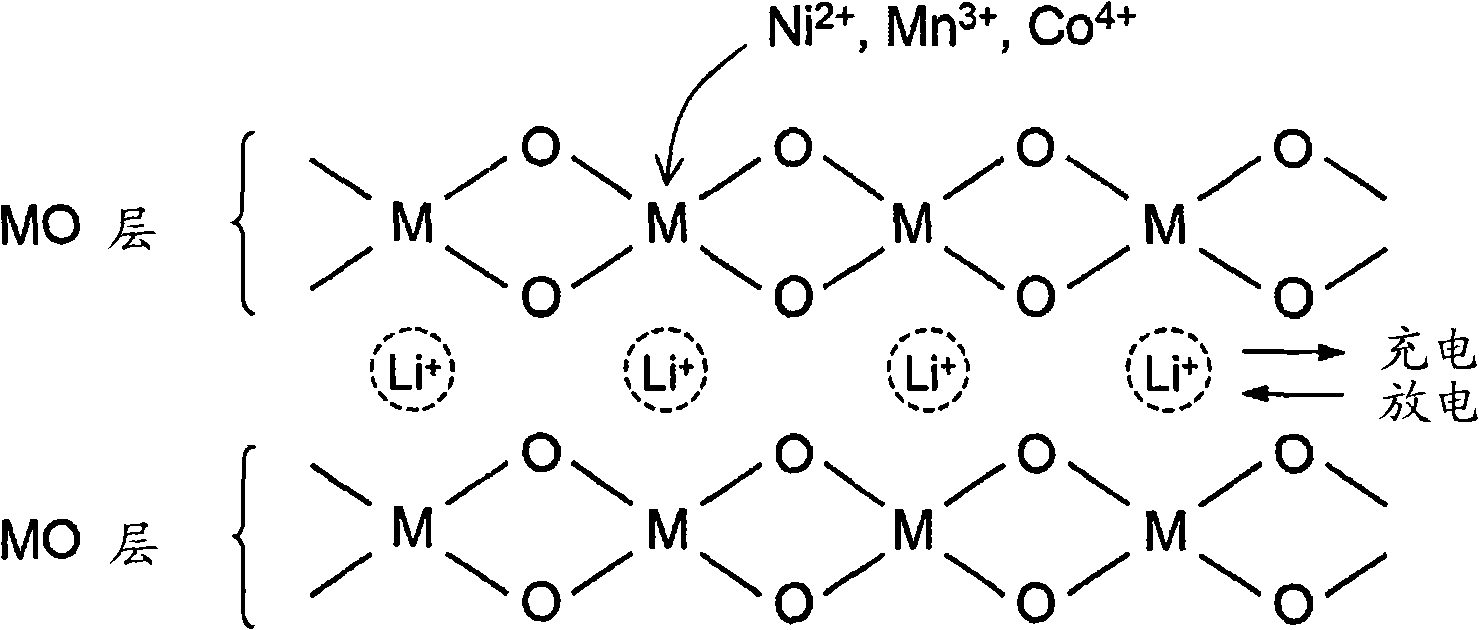
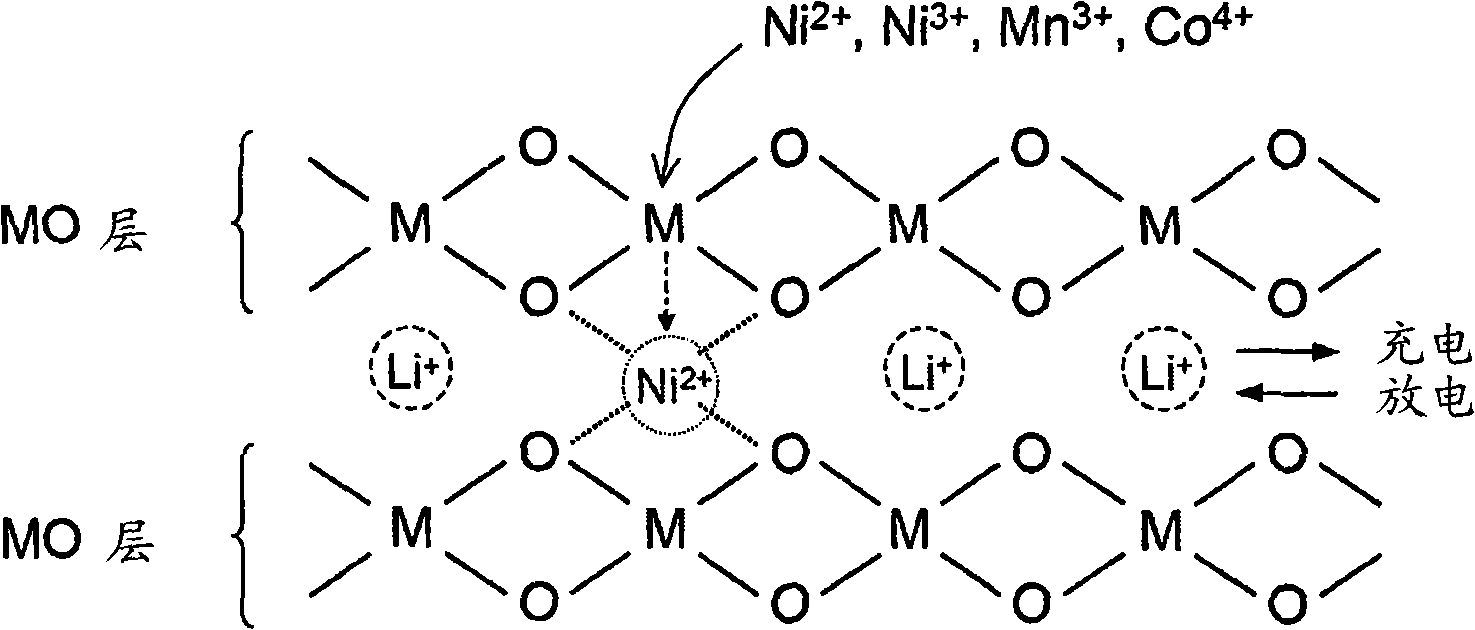
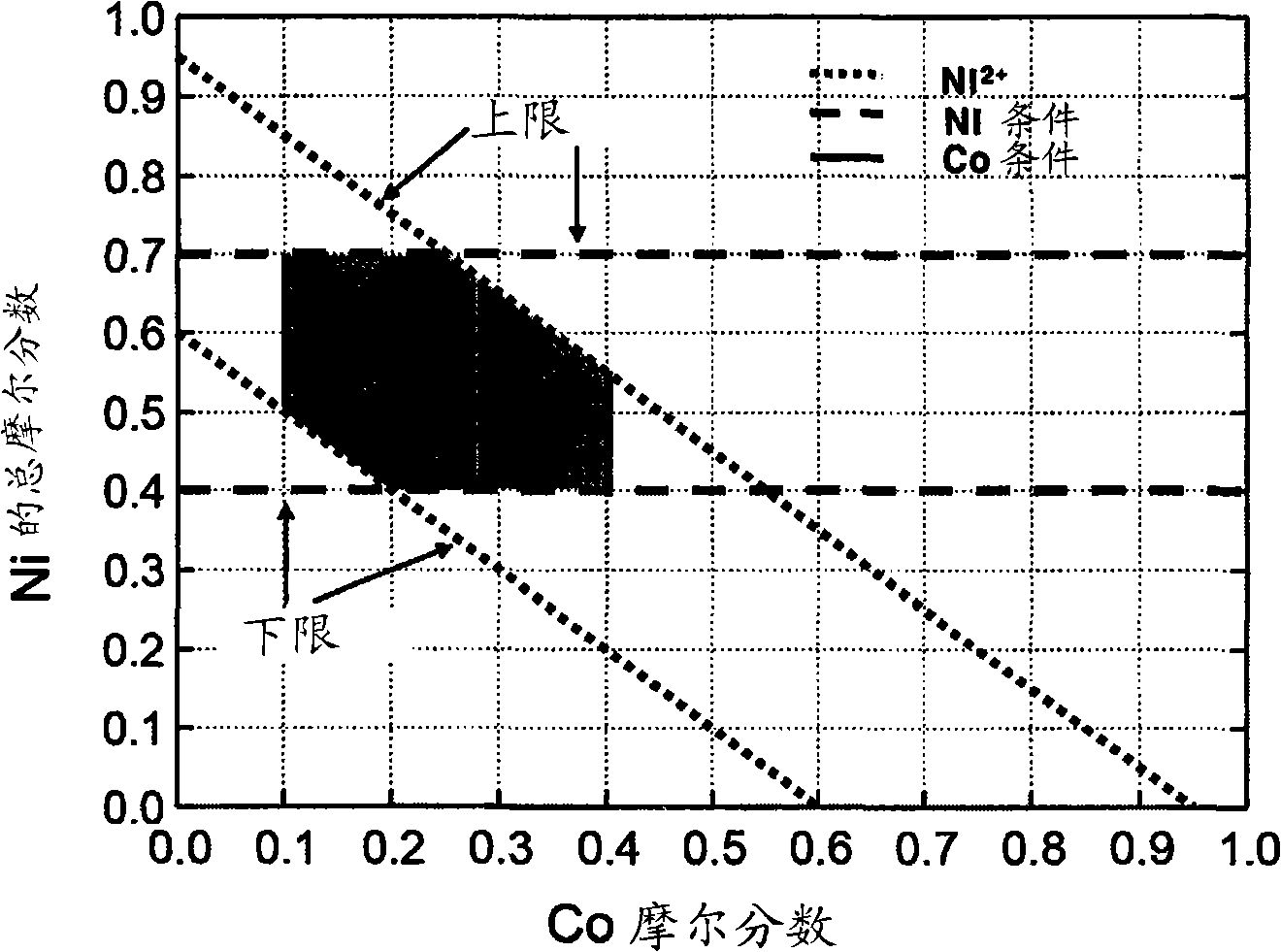
Material for lithium secondary battery of high performance
A lithium layer, mixed lithium technology, applied in secondary batteries, lithium storage batteries, battery electrodes, etc., can solve the problems of structural expansion, deterioration of cycle characteristics, deterioration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] A formula MOOH (M=Ni 4 / 15 (Mn 1 / 2 Ni 1 / 2 ) 8 / 15 Co 0.2 ) mixed hydroxides as mixed transition metal precursors, with Li 2 CO 3 Mixing in a certain stoichiometric ratio (Li:M=1.02:1) and sintering the mixture in air at various temperatures between 850 and 1000°C for 10 hours to obtain a lithium-mixed transition metal oxide. Here, the secondary particles maintain an intact shape without collapse, and the crystal size becomes larger as the sintering temperature increases.
[0117] From the results of X-ray analysis, it was confirmed that all samples had a good layered crystal structure. In addition, the unit cell volume did not change significantly with increasing sintering temperature, thus indicating no apparent oxygen deficiency and no apparent increase in cation mixing, and essentially no lithium evaporation.
[0118] The crystallographic data of the thus prepared mixed lithium transition metal oxide is shown in Table 1 below, and its FESEM image is as follows ...
Embodiment 2
[0133] Samples of mixed lithium transition metal oxides were subjected to pH titration prior to exposure to moisture as in Example 2 and stored at 60°C in air in a wet chamber (90% RH) for separate storage 17 hours and 3 days. The result obtained in this way is Figure 9 shown. The mixed lithium transition metal oxide of Example 2 (see Figure 9 ) and the sample of Comparative Example 3 (see Figure 8 ) for comparison, the sample of Comparative Example 3 (storage for 17 hours) consumed about 20 mL of HCl, while the sample of Example 2 (storage for 17 hours) consumed 10 mL of HCl, thus indicating that the water-soluble base produced was reduced by about half. In addition, in the samples stored for 3 days, the sample of Comparative Example 3 consumed about 110 mL of HCl, while the sample of Example 2 consumed 26 mL of HCl, which corresponds to a reduction of water-soluble base produced by about one-fifth. Therefore, it can be seen that the decomposition rate of the sample of...
Embodiment 3
[0137] put a Li 2 CO 3 And formula MOOH (M=Ni 4 / 15 (Mn 1 / 2 Ni 1 / 2 ) 8 / 15 Co 0.2 ) The mixture of mixed hydroxides was introduced into a furnace of about 20L chamber and sintered at 920°C for 10 hours, during which time more than 10m 3 The air was pumped into the furnace, from which about 5 kg of LiNiMO was prepared in one batch 2 .
[0138] After the sintering is completed, the unit cell constant is determined by X-ray analysis, and the unit cell volume is compared with the target value (Sample B of Example 1: 33.921 )Compare. ICP analysis confirmed that the Li to M ratio was very close to 1.00, and the unit cell volume distribution was within the target range. Figure 10 SEM images of the cathode active material thus prepared are shown, and Figure 11 Rietveld actuarial results are shown. As shown in these figures, it can be confirmed that this sample has high crystallinity and a good layered structure, and the Ni intercalated in the reversible lithium layer 2+ T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com