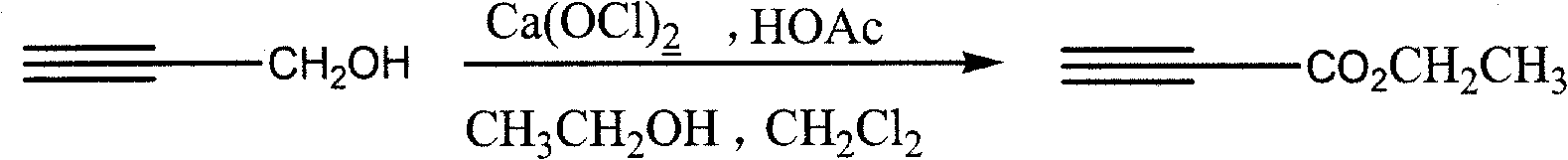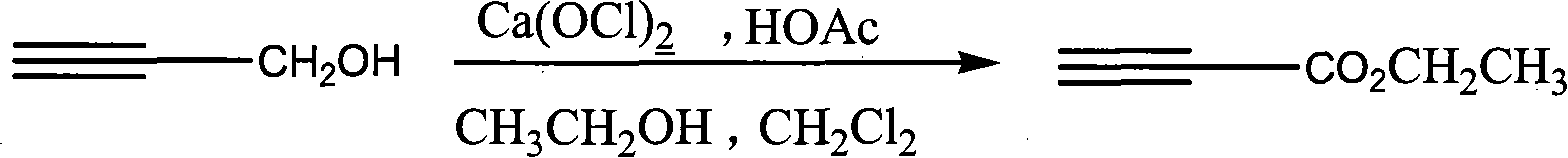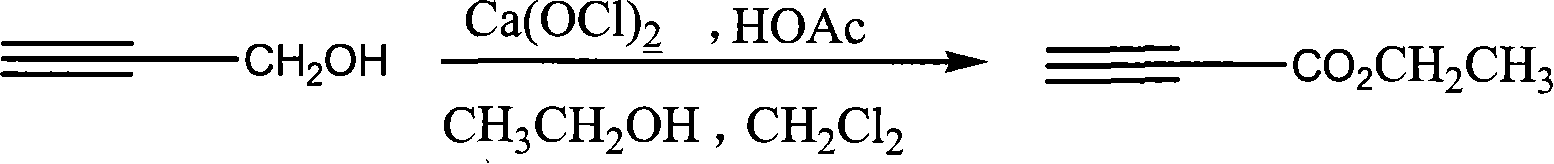Process for synthesizing ethyl propiolate
A technique for the synthesis of ethyl propiolate and its synthesis method, applied in the field of synthesis of ethyl propiolate, capable of solving problems such as operational hazards, environmental pollution, complex reaction systems, etc., achieving mild reaction conditions, no pollution to the environment, and easy availability of raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] The synthetic route of the present invention is:
[0021]
[0022] The possible mechanism of the method is: calcium hypochlorite reacts with acetic acid to generate oxidative hypochlorous acid, the hypochlorous acid preferentially oxidizes propynyl alcohol to generate propiole aldehyde, and propiole aldehyde is further converted into ethyl propiolate.
[0023] The present invention will be described in detail below in conjunction with specific embodiments.
[0024] In a 500mL reaction flask, put 230ml of dichloromethane, 56g of propynyl alcohol, 138g of absolute ethanol, 360g of glacial acetic acid, 429g of calcium hypochlorite and 20g of molecular sieves, and stir at room temperature. GC followed until the starting material was reacted to completion. After the reaction, add 300ml of water and 10g of sodium thiosulfate and stir for half an hour, then slowly add solid sodium bicarbonate to adjust the pH to 7-8. Stand to separate the layers, extract the aqueous phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com