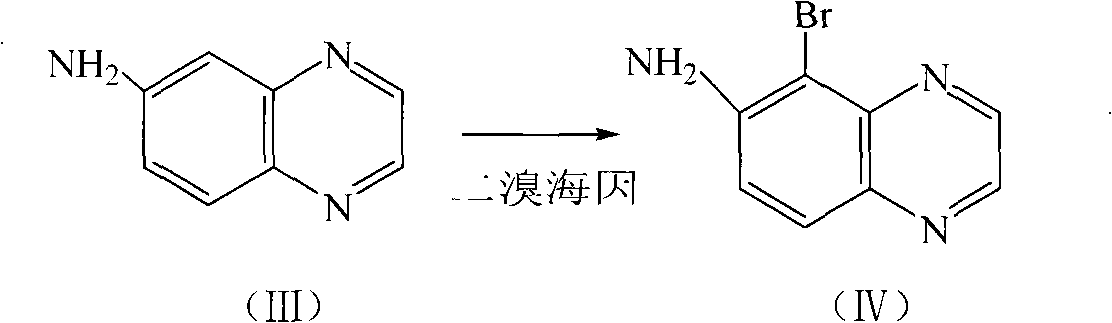
Method for preparing medicament midbody 5-bromine-6-amido quinoxaline
A technology of aminoquinoxaline and nitroquinoxaline, which is applied in the field of preparation of 5-bromo-6-aminoquinoxaline, an intermediate of brimonidine tartrate, a glaucoma drug, can solve the problem of volatile, low yield, iron Ion residues and other problems, to achieve the effect of being conducive to industrial production, high product yield, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
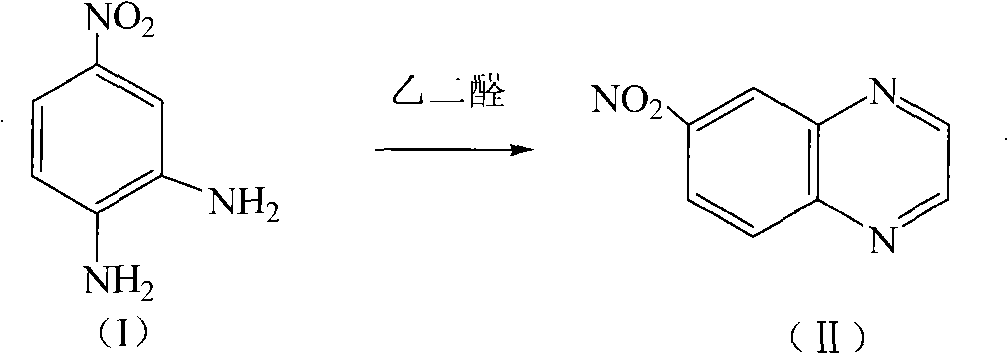
[0028] Embodiment 1: the preparation of 6-nitroquinoxaline
[0029] Glyoxal (40% aqueous solution, 74ml) was added dropwise to 600ml aqueous solution of 39.25g of 4-nitrophenylenediamine, after the dropping was completed, it was heated to 100°C under nitrogen protection, and kept for 5 hours. TLC tracking detection, cooling, filtration, washing with water, extraction with dichloromethane, drying over magnesium sulfate and precipitation to obtain 43.5 g of orange solid (m.p. 177°C, yield 97.5%).
Embodiment 2
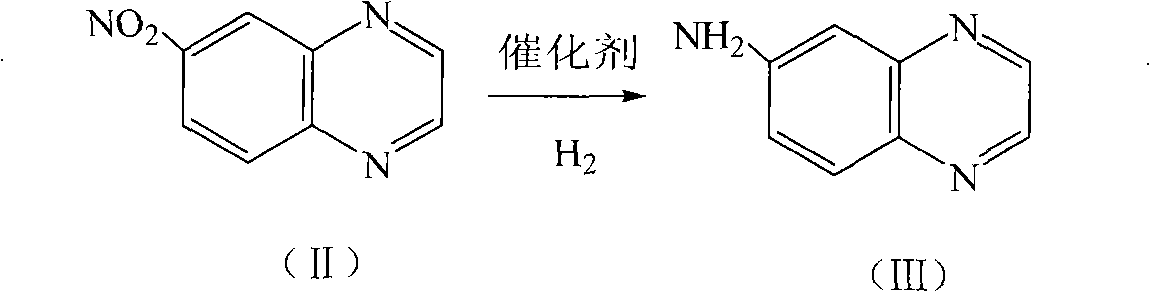
[0030] Embodiment 2: the preparation of 6-aminoquinoxaline
[0031] 100ml methanol as solvent, mix 10g (II) and 0.5g palladium-carbon catalyst (5%), add to a 250ml autoclave, replace with hydrogen, feed hydrogen, the temperature is about 70°C, the pressure is 2MPa, after about three hours, It was detected by liquid chromatography that the reaction was complete, taken out, filtered, washed, and the brown-red filtrate was precipitated to obtain a brick-red powdery solid, which was recrystallized from toluene to obtain 6.9 g of a yellow solid, with a yield of 83%.
Embodiment 3
[0032] Embodiment 3: the preparation of 6-aminoquinoxaline
[0033] 100ml of ethanol was used as solvent, mixed with 10g (II) and 0.3g palladium carbon catalyst (5%), added to a 250ml autoclave, replaced by hydrogen, fed with hydrogen, the temperature was about 80°C, and the pressure was 2MPa. After about three hours, It was detected by liquid chromatography that the reaction was complete, taken out, filtered, washed, and the brown-red filtrate was precipitated to obtain a brick-red powdery solid, which was recrystallized from toluene to obtain 6.2 g of a yellow solid, with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com