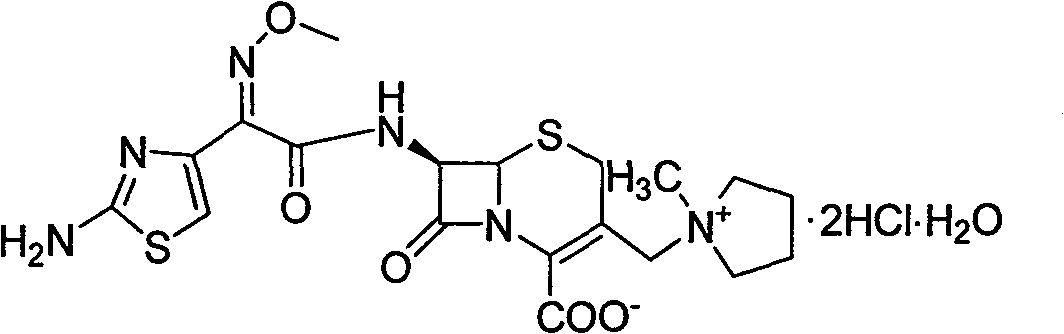
Method for synthesizing antibiotic cefepime hydrochloride
A technology of cefepime hydrochloride and a synthetic method, which is applied in antibacterial drugs, organic chemistry, etc., can solve problems such as harsh reaction conditions, high raw material costs, and long reaction time, and achieve simple process conditions, stable product quality, and product yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
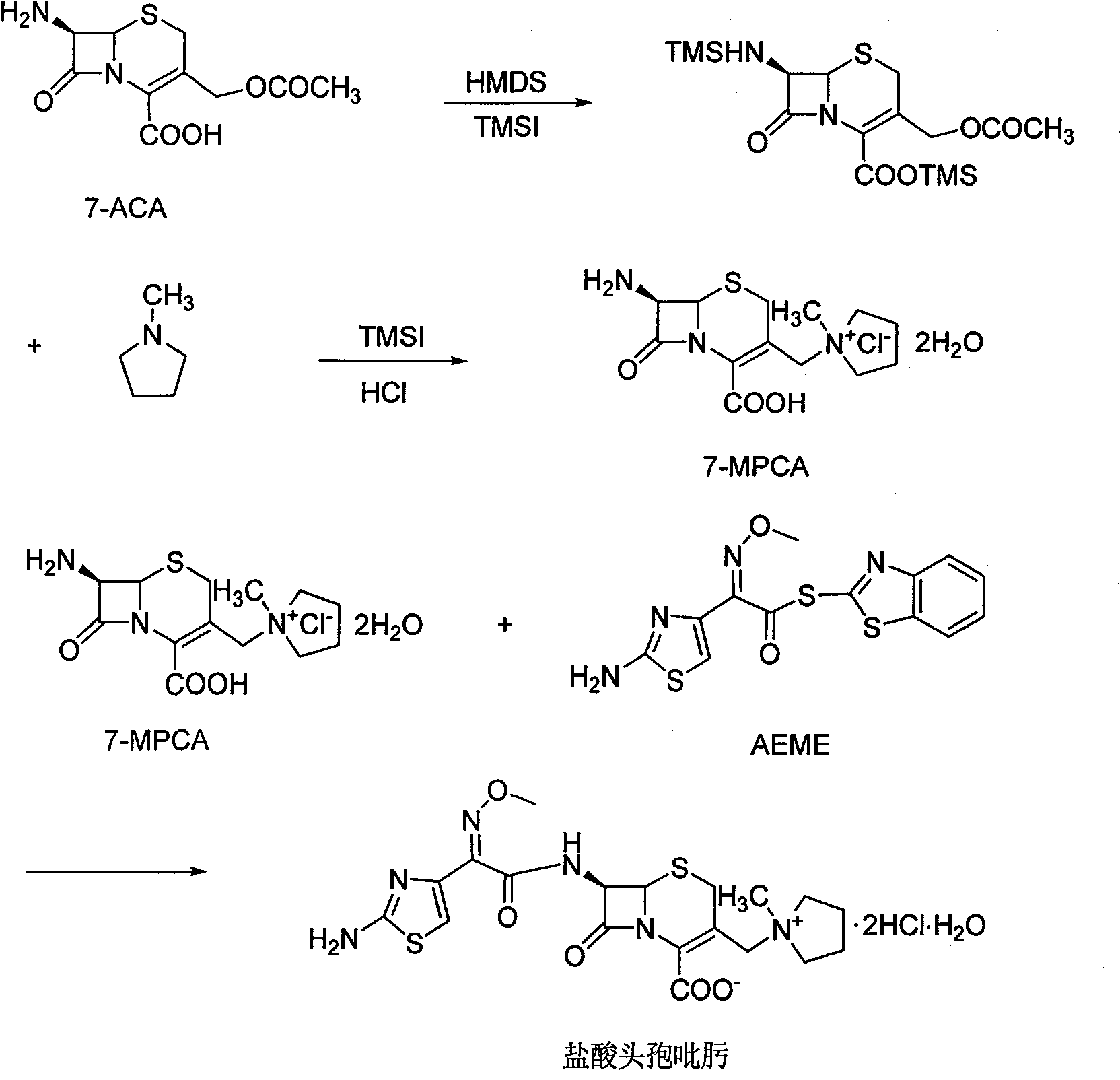
[0024] The synthesis of embodiment 1 7-MPCA
[0025] In the flask, add 40g (0.14mol) 7-aminocephalosporanic acid (7-ACA), 27.4g (0.17mol) hexamethyldisilane, 240mL dichloromethane, and stir at 30-35°C for 6-7 hours . Then the temperature was lowered to -10°C, 27 mL of N,N-diethylaniline was added, 61.2 g (0.3 mol) of iodotrimethylsilane was added dropwise, and the reaction was stirred for 1-2 hours.
[0026] Then, 20.4 g (0.24 mol) of N-methylpyrrolidine was added dropwise. After the dropwise addition was completed, the mixture was incubated for 1 hour, and then naturally raised to room temperature for overnight reaction.
[0027] After the reaction, add 50mL of isopropanol + 160mL of concentrated hydrochloric acid + 300mL of deionized water mixture dropwise. During the dropwise addition, control the temperature of the feed liquid to not exceed 15°C, and stir at this temperature for 10 minutes until the solid is completely dissolved. Then the liquids were separated, the orga...
Embodiment 2
[0029] The preparation of embodiment 2 cefepime hydrochloride
[0030] In the flask, add 550mL of deionized water, 250mL of DMF, 39g of 7-MPCA and 80g of AE active ester, then cool to -5°C, and add 37mL of triethylamine dropwise. During the dropping process, the temperature does not exceed 0°C. After the dropwise addition was complete, stirring was continued for 1 hour. Then the temperature was raised to 15-20° C. for 6 hours, and the reaction solution was extracted three times with 600 mL of dichloromethane. The organic phase was back-extracted with 300 mL deionized water, and the aqueous phase was combined, and the aqueous phase was decolorized with activated carbon.
[0031] The aqueous phase was quickly filtered through an aluminum oxide column, then added 40.6mL of 6mol / L hydrochloric acid, stirred at room temperature for 1 hour, then added 2.5L of acetone, stirred for 4 hours, filtered, the filter cake was washed with acetone, and dried 52.6 g of cefepime hydrochloride...
Embodiment 3
[0035] 7-ACA is placed in the organic solvent N, N-dimethylformamide (DMF) or N, N-dimethylacetamide (DMAC), and 1 times of hexamethyldisilazane is added to the amount of 7-ACA and 0.1% iodotrimethylsilane in the amount of 7-ACA, and reflux for 2 hours to obtain an orange-yellow reaction solution, cool to room temperature, add trimethyliodosilane dropwise, then add N-methylpyrrolidine dropwise, and then Solution to obtain 7-MPCA; The weight ratio of 7-ACA and organic solvent is 1:1.
[0036] The obtained 7-MPCA and AE active ester are mixed and placed in a mixed solvent with a volume ratio of 1:5 of an organic solvent and water, and in a mixed solvent of an organic solvent and water, adjust the pH with alkali sodium hydroxide or potassium hydroxide When the value reaches 7.0, it dissolves and undergoes an acylation reaction to obtain a cefepime solution, and the temperature of the acylation reaction is -10°C. The weight ratio of the 7-MPCA and AE active ester is 1:1.5, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com