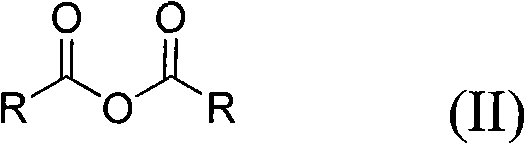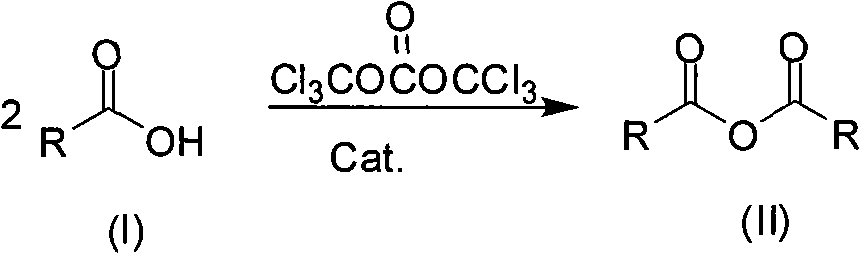Method for preparing symmetrical acid anhydride
A symmetrical, acid anhydride technology, applied in the preparation of carboxylic acid anhydrides, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of unstable catalysts, troublesome post-processing, severe conditions, etc., to achieve good promotion and application prospects, and less catalyst consumption. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of propionic anhydride
[0024] The ratio of the amount of feed materials is carboxylic acid RCOOH: bis(trichloromethyl) carbonate: catalyst 1:0.17:0.01, RCOOH is propionic acid, catalyst is N,N-dimethylformamide, and organic solvent is ethyl acetate , Its dosage is 3 times the mass of carboxylic acid.
[0025] In a 250mL three-necked flask equipped with a thermometer, reflux condenser and mechanical stirring, 7.4g (100mmol) of propionic acid, 5.0g (17mmol) of bis(trichloromethyl) carbonate, 22.2ml of ethyl acetate and N, N -Dimethylformamide 0.07 g (1 mmol). After the addition, the temperature is increased to 60-65°C, and the reaction is kept for 6 hours. After the reaction is completed, the solvent is evaporated under reduced pressure, and then 6.3 g of propionic anhydride is obtained by high-vacuum distillation. The product yield is 97.0% as an oily substance. 1 H-NMR (400MHz, CDCl 3 ): 1.18 (6H, t, J = 3.6 Hz), 2.50 (4H, q, J = 6.8 Hz); IR (KBr, c...
Embodiment 2
[0026] Example 2: Preparation of benzoic anhydride
[0027] The ratio of the amount of feed materials is carboxylic acid RCOOH: bis(trichloromethyl) carbonate: catalyst 1:0.17:0.005, RCOOH is benzoic acid, the catalyst is N,N-dimethylformamide, and the organic solvent is toluene. The dosage is twice the mass of carboxylic acid.
[0028] In a 250mL three-necked flask equipped with a thermometer, reflux condenser and mechanical stirring, add 12.2g (100mmol) of benzoic acid, 17g (17mmol) of bis(trichloromethyl) carbonate, 24.4ml of toluene and N,N-dimethyl Benzamide 0.04g (0.5mmol). After the addition, the temperature is increased to 70-75°C, and the reaction is kept for 5 hours. After the reaction is completed, the solvent is evaporated under reduced pressure, and the residue is recrystallized with cyclohexane to obtain 11.0 g of benzoic anhydride, with a product yield of 97.0%, solid. Melting point: 43-44°C. 1 H-NMR (400MHz, CDCl 3 ): 8.12 (4H, d, J=8.4 Hz, ArH), 7.68-7.58 (2H, m, ...
Embodiment 3
[0029] Example 3: Preparation of o-chlorobenzoic anhydride
[0030] Use o-chlorobenzoic acid instead of benzoic acid, the reaction solvent is dichloromethane, the reaction temperature is 40~45℃, the reaction time is 10h, the operation process is the same as that of Example 2, to obtain o-chlorobenzoic anhydride, the yield is 94%, solid . Melting point: 79-81°C. 1 H-NMR (400MHz, CDCl 3 ): 8.04 (dd, 2H, J=2, 16Hz), 7.54-7.48 (4H, m), 7.42-7.26 (2H, m); IR (KBr, cm -1 ): 1780, 1718; MS (EI): m / z(%)=259(M+1,56), 141(39), 139(100), 113(17), 111(44), 75(18 ). 13 C NMR(100MHz, CDCl 3 ): 160.34, 135.1, 134.2, 132.6, 131.6, 127.8, 126.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com