Quality control method of compound inflammation-diminishing and gallbladder-benefiting formulation
A technology of compound anti-inflammatory and choleretic, quality control method, applied in digestive system, measuring device, pharmaceutical formula, etc., can solve the problems of backward quality control and identification method of compound anti-inflammatory and choleretic preparation, unable to comprehensively evaluate the quality of compound anti-inflammatory and choleretic preparation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
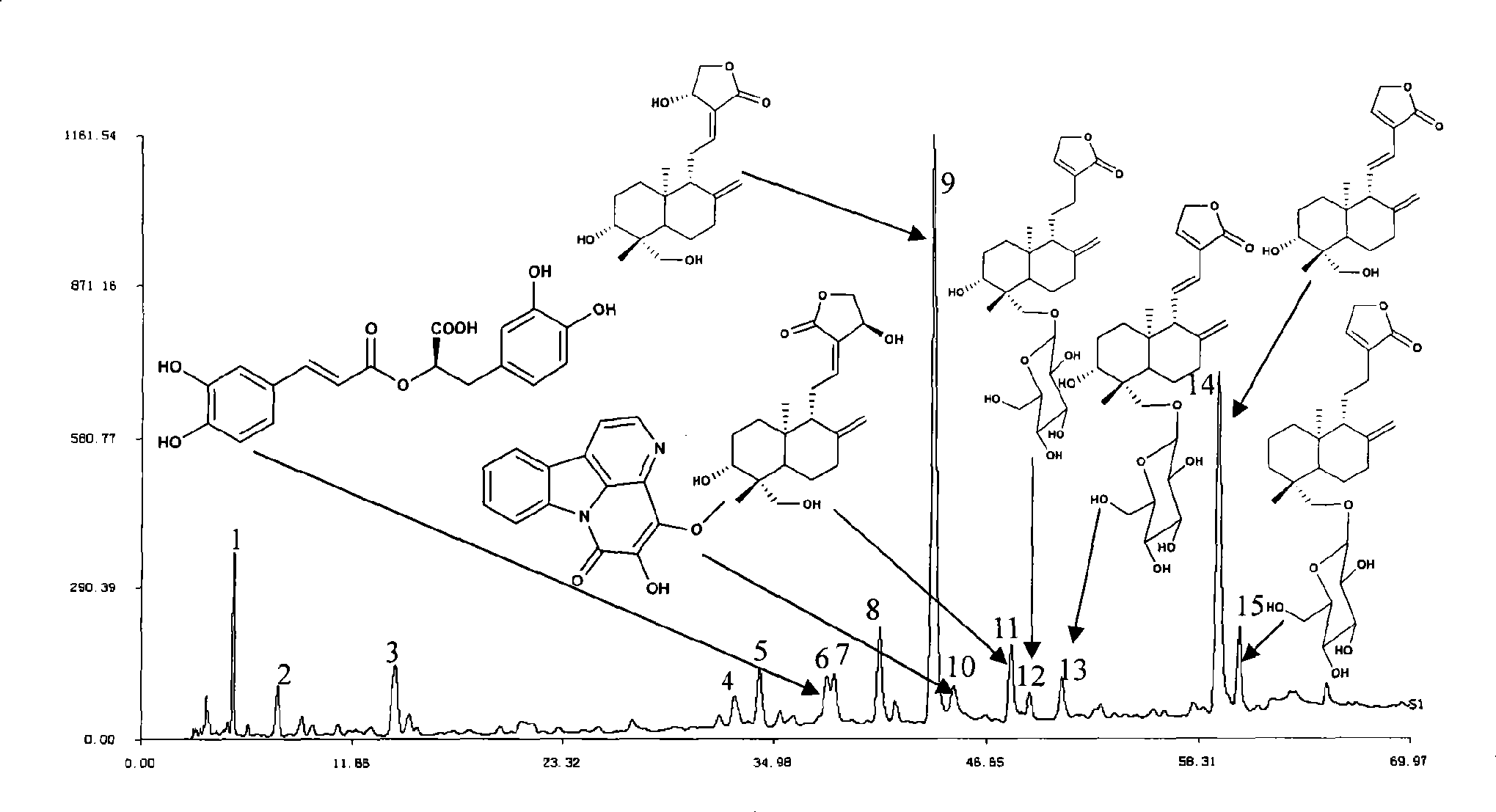
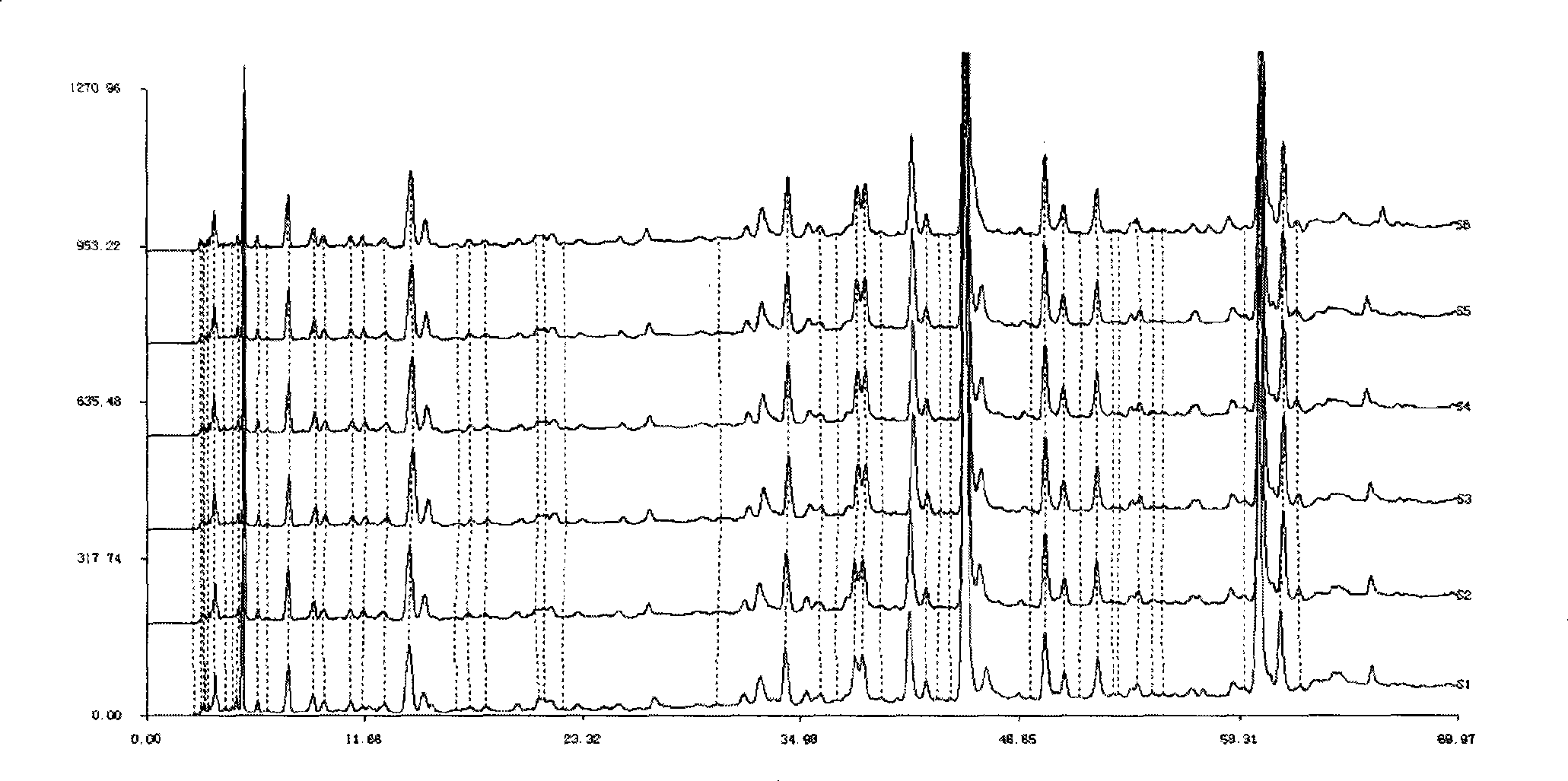
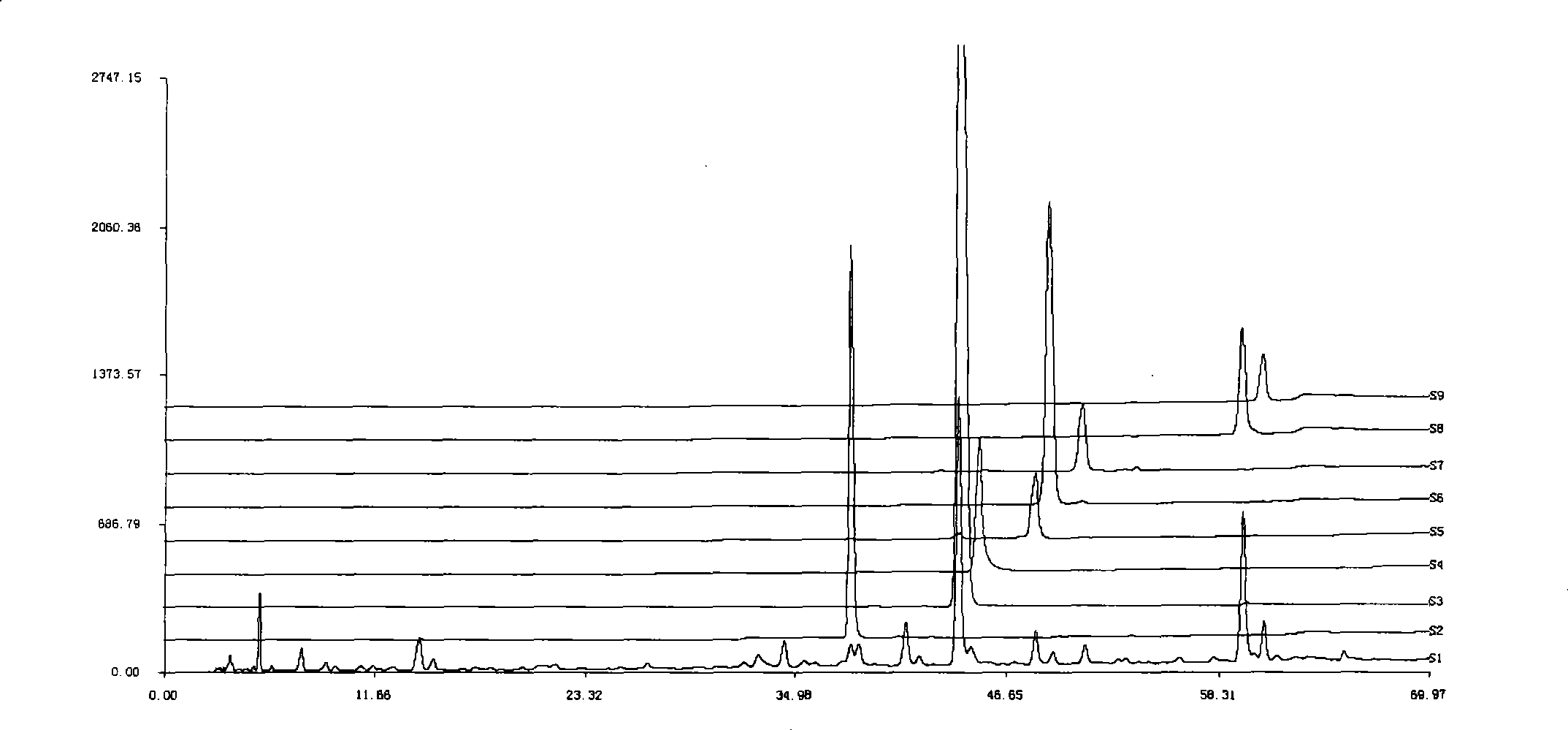
[0071] Example 1 Establishment of HPLC standard fingerprint of compound anti-inflammatory and choleretic preparation
[0072] Take 150g each of Andrographis paniculata and Bitterwood, chop and mix, heat and extract twice with 80% to 85% ethanol, filter, recover ethanol from the filtrate and concentrate it into a thick paste to obtain 43.5g of extract; take 150g of Xihuangcao and cut into pieces , add water to decoct twice, combine the filtrate, filter, the decoction is concentrated to an appropriate amount, add 5 times the amount of 70% ethanol, shake up, leave standstill, filter, the filtrate reclaims ethanol and concentrates into a thick paste to obtain 9g extract.
[0073] Take 5g of the mixed thick paste of Andrographis paniculata and bitter wood and 1.03g of Xihuangcao thick paste respectively, combine them, add 100mL of 70% methanol, dissolve them by ultrasonic, take 4mL and pass through a 0.45μm microporous membrane, and then take 1.5mL of the filtrate and use it with so...
Embodiment 2
[0085] Example 2 Separation and structural identification of chromatographic peaks
[0086] The three traditional Chinese medicines that make up the compound anti-inflammatory and choleretic preparation were extracted and separated respectively: Andrographis paniculata, Bitterwood, and Xihuangcao.
[0087] Take Andrographis paniculata 60% ethanol extract 500g, add water to suspend, extract with cyclohexane, ethyl acetate, n-butanol respectively. Carry out silica gel column separation to ethyl acetate extract part, carry out gradient elution with chloroform:methanol solvent system (100:0,99:1,98:2,95:5,90:10,80:20,50:50 , 0:100). The eluted similar fractions were combined to obtain ten fractions from APE-1 to APE-10. Each fraction was subjected to repeated silica gel column chromatography, and compounds II, IV to VIII were isolated and obtained.
[0088] Get Xihuangcao 60% ethanol extract 600g, add water to suspend, carry out macroporous resin HP-20 column separation to it, ...
Embodiment 3
[0111] Example 3 Inhibitory activity of andrographolide on release of NO, TNF-α and IL-6 from mouse mononuclear macrophage RAW 264.7 induced by lipopolysaccharide
[0112] 1. Inhibitory activity of lipopolysaccharide-induced release of nitric oxide (NO) from mouse macrophages
[0113] Mouse mononuclear macrophage RAW 264.7 (ATCC TIB-71) was cultured in 10% heat-inactivated (56°C, 30min) fetal bovine serum (FBS), 100 U / mL penicillin sodium (Gibco), 100 μg / mL streptavidin in RPMI 1640 (Gibco) medium, 37°C, 5% CO 2 grown in a constant temperature incubator.
[0114] Because NO is extremely unstable, it is quickly metabolized into nitrous acid (NO) in the cell culture supernatant. 2 - ), so the Griess method was used to measure NO in the sample 2 - concentration as an indicator of NO levels. Griess reagent A: 0.1% N-naphthalene diamine dihydrochloride dissolved in water; Griess reagent B: 1% sulfanilamide dissolved in 5% H 3 PO 4 middle. Mix reagents A and B in equal volu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com