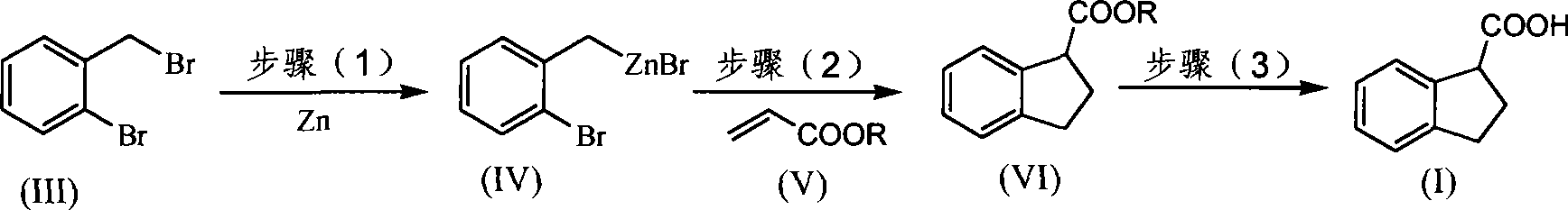
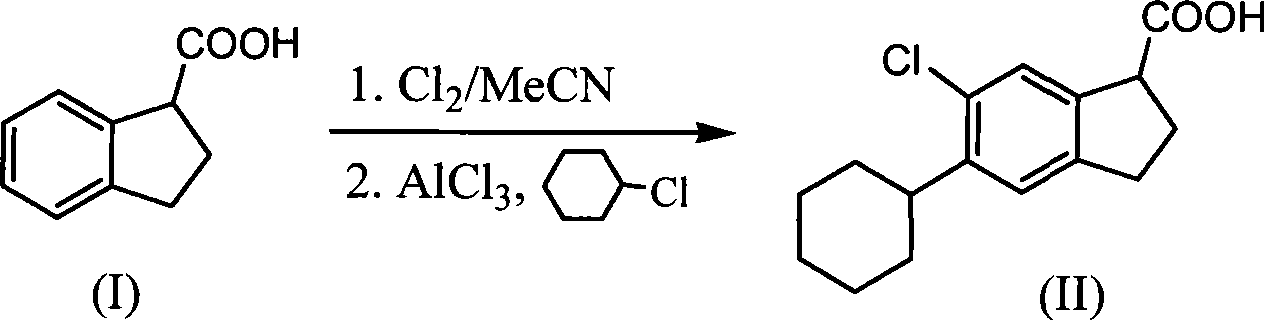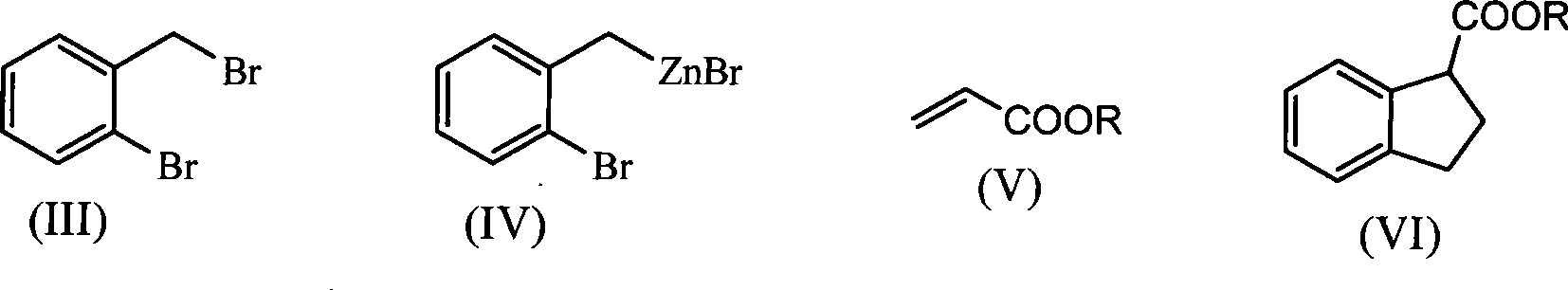Method for synthesizing raw medicine dihydroindene-1-carboxyl acid for clidabac
A technology of carboxylic acid and indane, which is applied in the field of indane-1-carboxylic acid synthesis, can solve the problems of high production cost and long process route, and achieve the effect of reducing production cost and shortening the synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0038] Synthesis of indane-1-carboxylic acid (I) from indane-1-carboxylic acid methyl ester (VIa)
[0039] (1) Synthesis of indane-1-carboxylic acid methyl ester (VIa) (chemical name 1-formyl methyl-2,3-dihydro-1-hydrogen-indene) under nitrogen atmosphere, continuously stirred at 8°C Under certain conditions, 50 mL of tetrahydrofuran solution dissolved in 17.5 g o-bromobenzyl bromide (70 mmol) was added dropwise to tetrahydrofuran (20 mL) solution containing 6.7 g of zinc powder (105 mmol), and reacted for 30 h. After the reaction is completed, the o-bromobenzyl zinc reagent can be obtained, which is sealed and stored for later use.
[0040] In another container, under the same nitrogen atmosphere and constant stirring, add the catalyst Ni(dppf) 2 Cl 2 (0.1mmol) and 10mL tetrahydrofuran as a solvent, then add methyl acrylate (Va) 87mg (1mmol), heat the oil bath to reflux, then dropwise add 3.5mL zinc reagent (3.5mmol), after reacting for 10 hours, the reaction system was coo...
example 2
[0044] Synthesis of indane-1-carboxylic acid (I) from ethyl indane-1-carboxylate (VIb)
[0045] (1) Indane-1-carboxylate ethyl ester (VIb) (chemical name: 1-formyl ethyl-2,3-dihydro-1-hydro-indene) was synthesized under nitrogen atmosphere, continuously stirred at 0°C Under the conditions, 50 mL tetrahydrofuran solution dissolved in 17.5 g o-bromobenzyl bromide (70 mmol) was added dropwise to tetrahydrofuran (50 mL) solution containing 8.9 g zinc powder (140 mmol), and reacted for 10 h. After the reaction is completed, the o-bromobenzyl zinc reagent can be obtained, which is sealed and stored for later use.
[0046] Under nitrogen atmosphere and constant stirring, the catalyst Ni{P(p-MeOC 6 h 4 ) 3} 2 I (0.2mmol) and 15mL ether were used as solvents, then 101mg (1mmol) of ethyl acrylate (Vb) was added, the oil bath was heated to reflux, then 2.8mL zinc reagent (2mmol) was added dropwise, and after 10 hours of reaction, the reaction system was cooled To room temperature, a...
example 3
[0050] Synthesis of indane-1-carboxylic acid (I) from butyl indane-1-carboxylate (VIc)
[0051] (1) Synthesis of butyl indane-1-carboxylate (chemical name 1-butyl carboxylate-2,3-dihydro-1-hydrogen-indene)
[0052] Under nitrogen atmosphere, under the condition of continuous stirring at 20°C, 50 mL of ethylene glycol dimethyl ether solution dissolved in 17.5 g of o-bromobenzyl bromide (70 mmol) was added dropwise to ethylene glycol dimethyl ether solution containing 5.1 g of zinc powder (80 mmol). Diethyl ether (90mL) solution, reacted for 20h. After the reaction is completed, the o-bromobenzyl zinc reagent can be obtained, which is sealed and stored for later use.
[0053] Under nitrogen atmosphere and constant stirring, the catalyst Ni{P(p-F 3 CC 6 h 4 ) 3} 2 Cl 2 (0.3mmol) and 20mL trifluorotoluene as solvent, then add butyl acrylate (Vc) 129mg (1mmol), heat the oil bath to reflux, then add 10mL zinc reagent (5mmol) dropwise, after reacting for 30 hours, the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com