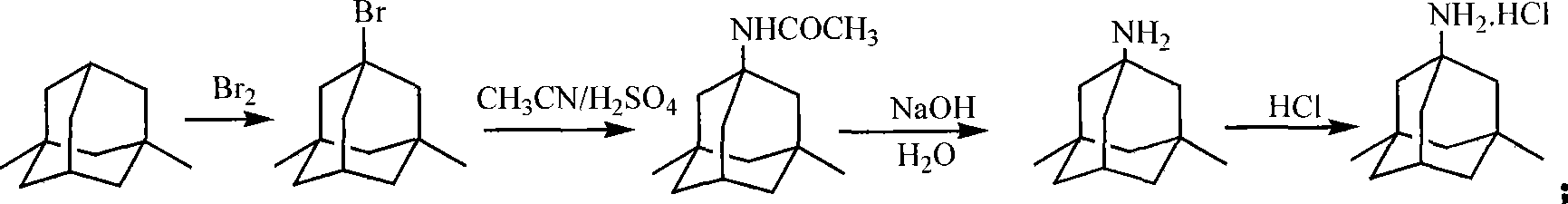
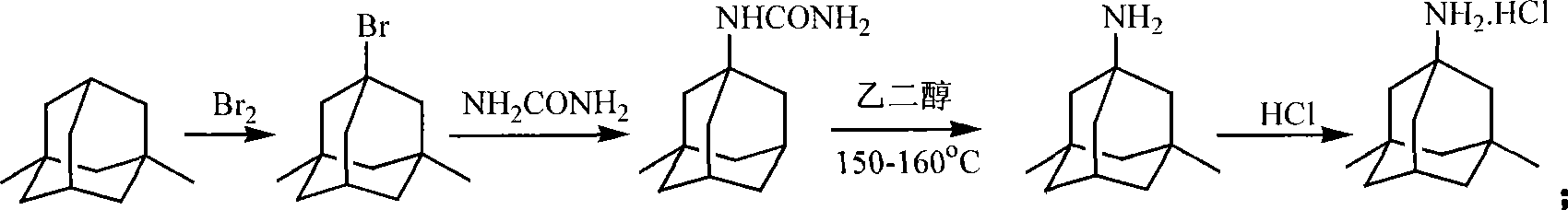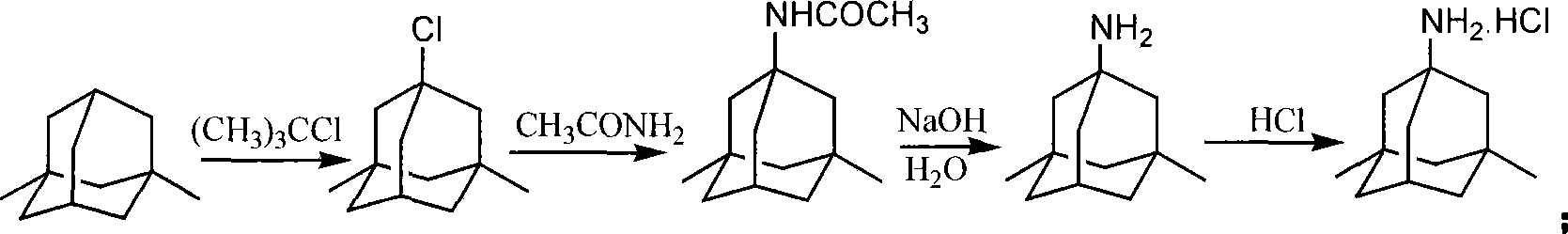Method for synthesizing memantine hydrochloride
A technology of memantine hydrochloride and dimethyladamantane, which is applied in the directions of drug combination, amino-substituted functional group preparation, nervous system diseases, etc., can solve the problems of insufficiency, severe reaction, combustion and explosion accidents, etc., to avoid production reduction, Safe and simple operation, avoid the effect of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) Take 3.8mL of 1,3-dimethyladamantane (0.02mol), 1.35mL of concentrated nitric acid (0.03mol), 0.334g of N-hydroxy-phthalimide (0.002mol), 5mL Acetic acid was put into a three-necked flask, and the oil bath was slowly heated to 70°C. After 10 hours of reaction, the pH was adjusted to 9 with saturated sodium carbonate solution, extracted three times with ethyl acetate (3×10 mL), and the organic phase was washed with water until neutral. Dry over anhydrous magnesium sulfate. The solvent was evaporated to obtain 3.310 g of crude 1-nitro-3,5-dimethyladamantane;
[0048] (2) Add 10mL of ethanol to the round bottom flask, then add the reactant 1-nitro-3,5-dimethyladamantane crude product and 0.3g of 10% Pd-C obtained in step (1), and remove the air , feed hydrogen, stir at room temperature for 24 hours, filter, distill off ethanol, add petroleum ether to the residue, and filter off insoluble matter;
[0049] (3) Pass dry hydrogen chloride gas into the above-mentioned fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com