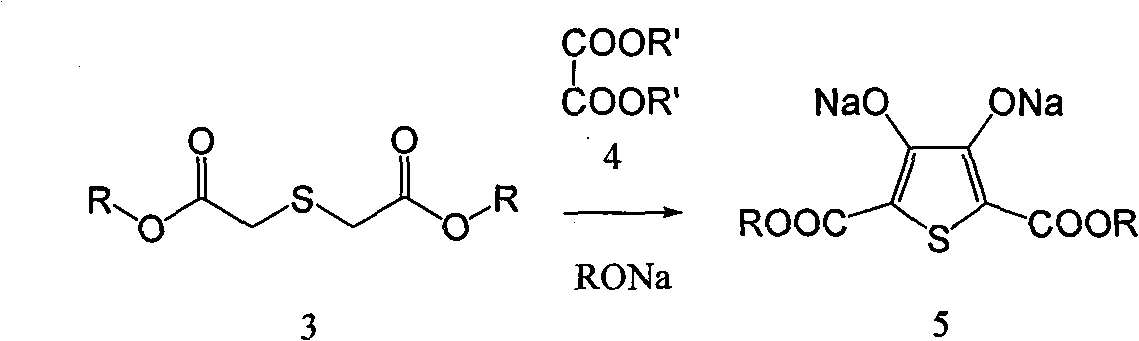
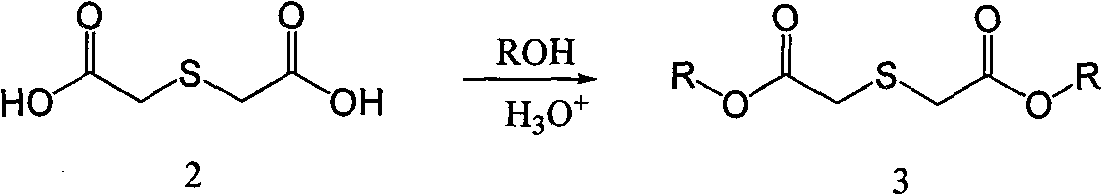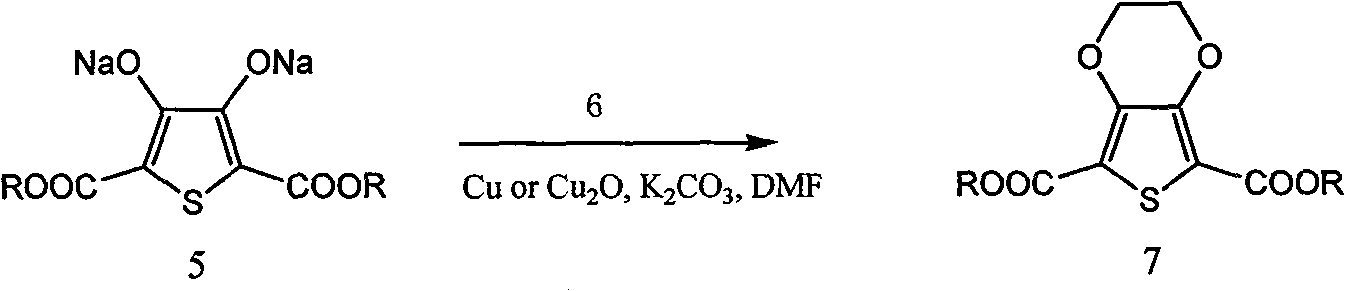Process for producing 3,4-enedioxy thiophene
An ethylenedioxythiophene and ethyl technology, applied in 3 fields, can solve the problems of dark color, high single impurity content, heavy odor, etc. of EDOT products, improve product color and storage stability, reduce single impurity content, increase Effect of Reaction Yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of 2,5-dicarboxylate-3,4-dihydroxy disodiumthiophene
[0054] 70g (0.466mol) thiodiglycolic acid, 1600mL methanol, 39g 98% sulfuric acid and a small amount of 4 Molecular sieves were put into a 2000mL three-necked flask, and heated to reflux for 1-4 hours. The temperature was lowered to 0°C, and 50 g of sodium carbonate was slowly added to adjust the pH of the system to above 7.0. Methanol was distilled off under reduced pressure, and 800 mL of methanol was distilled off, and the residual solvent moisture was detected to control the moisture content below 0.5%. Raise the temperature to 25°C, add 10 g of anhydrous calcium chloride, and stir for 20 minutes. Filter, collect the filtrate, and cool the filtrate to 0°C. Add 432g of 25% sodium methoxide (2mol), add dropwise 99.8g (0.63mol) of diethyl oxalate, and control the temperature at 0-5°C. After the dropwise addition was completed, the temperature was raised to reflux for 1 hour, and then cooled to 0°C....
Embodiment 2
[0056] Preparation of 2,5-Diethyl Dicarboxylate-3,4-Dihydroxy Disodium Thiophene
[0057] 70g (0.466mol) thiodiglycolic acid, 1700mL ethanol, 25g trifluoroacetic acid and a small amount of 4 Molecular sieves were put into a 2000mL three-necked flask, and heated to reflux for 1-2 hours. Cool down to 0°C, slowly add 100 g of sodium carbonate solution in batches, and adjust the pH of the system to above 7.0. Ethanol was distilled off under reduced pressure, and 700 mL of ethanol was distilled off, and the residual solvent moisture was detected to control the moisture content below 0.5%. Raise the temperature to 25°C, add 15g of anhydrous calcium chloride, and stir for 20 minutes. Filter, collect the filtrate, and cool the filtrate to 0°C. Add 680 g of 20% sodium ethoxide (2 mol), add dropwise 99.8 g (0.63 mol) of diethyl oxalate, and control the temperature at 0-5°C. After the dropwise addition was completed, the temperature was raised to reflux for 1 hour, and then cooled t...
Embodiment 3
[0059] Preparation of 2,5-Diethyl Dicarboxylate-3,4-Dihydroxy Disodium Thiophene
[0060] 140g (0.932mol) thiodiglycolic acid, 2500mL ethanol, 25g sulfuric acid and a small amount of 4 Molecular sieves were put into a 2000mL three-necked flask, and heated to reflux for 2 hours. The temperature was lowered to 0°C, and 544 g (1.6 mol) of sodium carbonate solution was slowly added in batches to adjust the pH of the system to above 7.0. Ethanol was distilled off under reduced pressure, and after 1000mL of ethanol was distilled off, the residual system solvent moisture was detected, and the moisture content was controlled to be below 0.5%. Warm up to room temperature, add 50 g of anhydrous calcium chloride, and stir for 30 minutes. Filter, collect the filtrate, and cool the filtrate to 0°C. Add 1360 g of 20% sodium ethoxide (4 mol), add dropwise 200 g (1.26 mol) of diethyl oxalate, and control the temperature at 0-5°C. After the dropwise addition was completed, the temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com