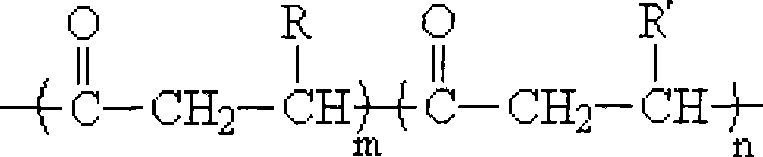
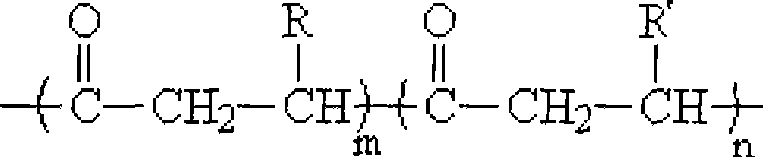Method for synthesizing poly hydroxy fatty acid ester block copolymers in situ
A technology of polyhydroxyalkanoate and block copolymers, which is applied in the field of preparation of polyhydroxyalkanoate block copolymers, can solve the problems of toxic solvents, etc., and achieve environmental friendliness, widely available raw materials, and simple synthesis process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] At 100°C, 30g of poly-3-hydroxybutyrate (number average molecular weight and molecular weight distribution were 4.5×10 4 and 4.8) were dissolved in a four-necked round-bottomed flask containing 300 mL of diethylene glycol dimethyl ether, condensed and refluxed, fed with nitrogen, and the stirring rate was 300 r / min. After the poly-3-hydroxybutyrate was completely dissolved, the liter Add 60mL of ethylene glycol and 0.3g of dibutyltin dilaurate at a high temperature to 140°C, add 0.3g of dibutyltin dilaurate at every hour after the reaction starts, and continue to pass nitrogen after 8 hours of reaction. , take out the reaction product, precipitate the product with ethanol, vacuum filter, and vacuum-dry at 65° C. to constant weight to obtain low-molecular-weight telechelic poly-3-hydroxybutyrate with hydroxyl end groups at both ends. Put telechelic poly-3-hydroxybutyrate and ε-caprolactone in a molar ratio of 1:20, add a total of 15g into a 250mL four-neck round bottom f...
Embodiment 2
[0032] At 100°C, 30g of poly-3-hydroxybutyrate (number average molecular weight and molecular weight distribution were 1.0×10 5 and 2.9) were dissolved in a four-necked round-bottomed flask containing 300 mL of diethylene glycol dimethyl ether, condensed and refluxed, fed with nitrogen, and the stirring rate was 200 r / min. After the poly-3-hydroxybutyrate was completely dissolved, the liter Add 60mL of ethylene glycol and 0.3g of dibutyltin dilaurate at a high temperature to 140°C, add 0.3g of dibutyltin dilaurate at every hour after the reaction starts, and continue to pass nitrogen after 10 hours of reaction. , take out the reaction product, precipitate the product with ethanol, vacuum filter, and vacuum-dry at 65° C. to constant weight to obtain low-molecular-weight telechelic poly-3-hydroxybutyrate with hydroxyl end groups at both ends. Put telechelic poly-3-hydroxybutyrate and D-lactide in a molar ratio of 1:15, add a total of 15g into a 250mL four-necked round-bottomed f...
Embodiment 3
[0035] At 100°C, 30g of poly-3-hydroxybutyrate (number average molecular weight and molecular weight distribution were 2.5×10 5 and 2.4) were dissolved in a four-neck round-bottomed flask with 300 mL of diethylene glycol dimethyl ether, condensed and refluxed, fed with nitrogen, and the stirring rate was 200 r / min. After the poly-3-hydroxybutyrate was completely dissolved, the liter Add 40mL of ethylene glycol and 0.3g of dibutyltin dilaurate at a high temperature to 140°C, add 0.3g of dibutyltin dilaurate at every hour after the reaction starts, and continue to pass nitrogen after 8 hours of reaction. , take out the reaction product, precipitate the product with ethanol, vacuum filter, and vacuum-dry at 65° C. to constant weight to obtain low-molecular-weight telechelic poly-3-hydroxybutyrate with hydroxyl end groups at both ends. Put telechelic poly-3-hydroxybutyrate and 1,5-dioxolan-2-one in a molar ratio of 1:18, add a total of 15g into a 250mL four-necked round-bottomed f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com