Agricultural bactericidal agent
An agricultural fungicide and a technology of bactericidal activity, applied in the field of pesticides, can solve the problems of crop injury and other problems, and achieve the effects of less injury, simple process, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
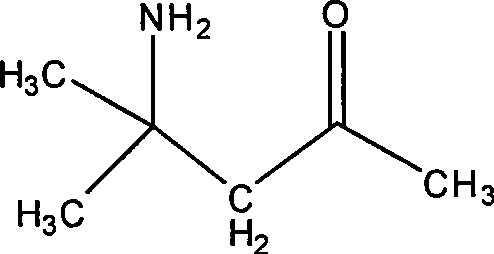
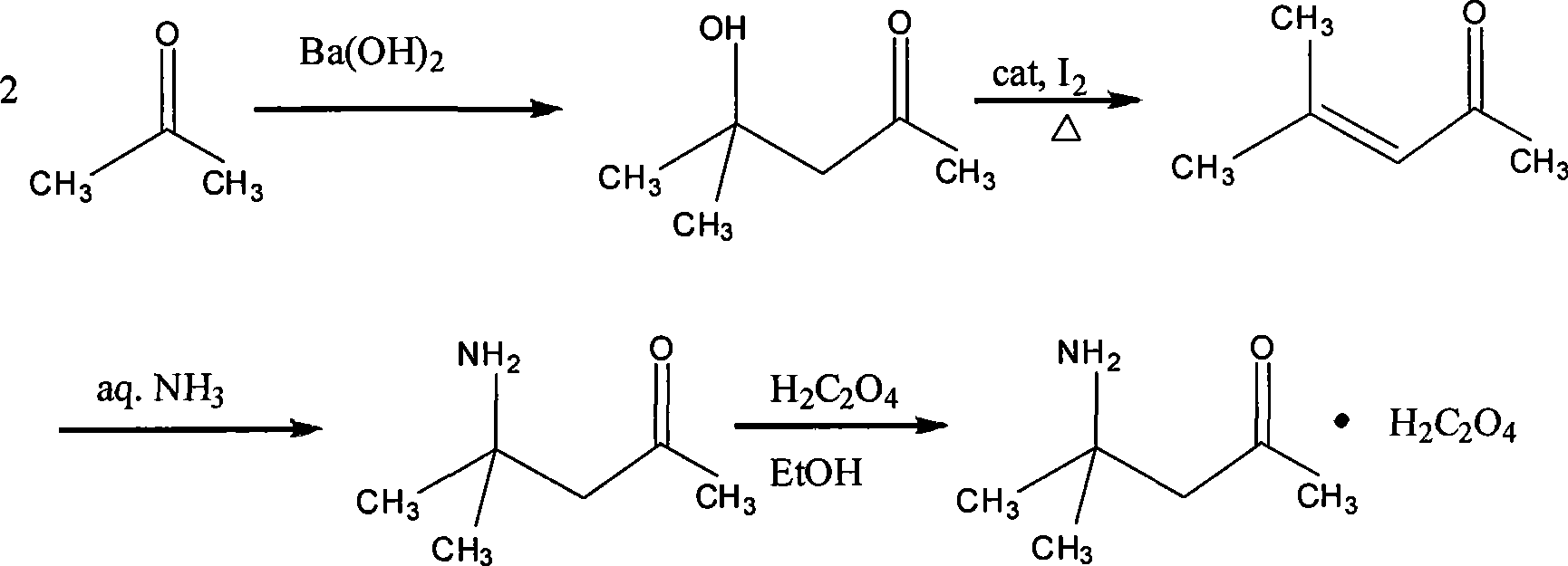
[0029] The synthesis of embodiment 1 diacetonamine oxalate
[0030] Add acetone (300mL, 4.1moles) and a small amount of zeolite into a 500mL round bottom flask, then connect the Soxhlet extractor with barium hydroxide in the extraction cylinder to the flask, and heat to reflux with an electric heating mantle. After 24 hours of reaction, the heat source was removed, and after the reaction solution was cooled, the Soxhlet extractor was removed, and about 100 mg of iodine was added to the flask, and a rectification column and a condenser were installed to carry out rectification, and three fractions were collected: I (56 ~ 80 ℃), this fraction is mainly acetone, containing a small amount of mesityl oxide; II (80 ~ 126 ℃), this fraction is divided into two phases after cooling, the lower phase is water, and the upper phase is mesityl oxide; III (126 ~131°C), the fraction is pure mesityl oxide. The acetone recovered in the I cut can be directly used for the next reaction, and the ...
Embodiment 2
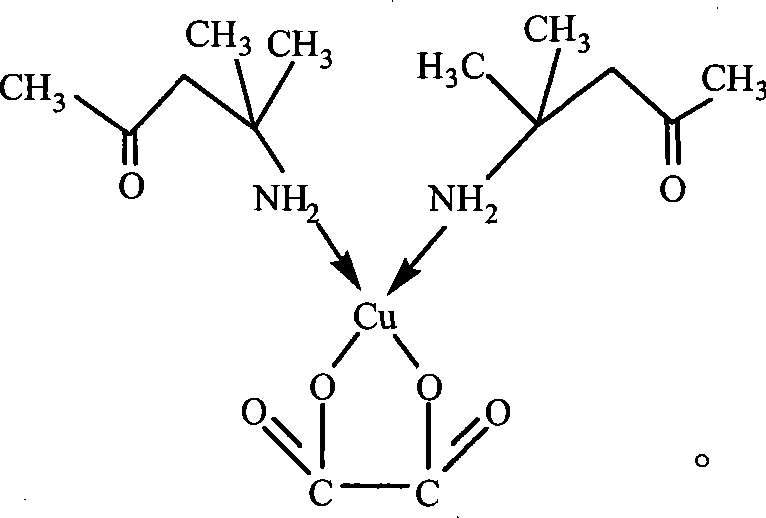
[0032] Embodiment 2 The preparation of diacetonamine copper oxalate
[0033] Weigh diacetonamine oxalate (2.5g, 0.012moles) into a 500ml round bottom flask, then add absolute ethanol (300ml) and a small amount of zeolite, heat until diacetonamine oxalate is completely dissolved, then add Cu(CH 3 COO) 2 ·H 2 O (2.0 g, 0.010 moles), reflux until the reaction solution is colorless, at this time, a sky blue crystal is formed at the bottom of the bottle, filter while hot, wash the crystal three times with hot absolute ethanol, and dry in the air.
Embodiment 3
[0034] Example 3 Determination of the in vitro antibacterial activity of diacetonamine copper oxalate on fungi
[0035] Using Zinc as the control agent, the antibacterial activity of diacetonamine copper complex of oxalate and diacetonamine on several tested pathogenic fungi was determined by the method of inhibiting spore germination. The results are shown in Table 1. It can be seen from Table 1 that the toxicity of the complex and the control agent Zinc to the six tested pathogenic bacteria is basically the same, while the toxicity of diacetonamine is much lower than these two agents. It can be seen that the antibacterial mechanism of diacetonamine oxalate and copper is obviously different after coordination. Diacetonamine mainly inhibits the growth of hyphae, while after coordination, it mainly inhibits spore germination.
[0036] Table 1 The virulence of diacetonamine copper oxalate in inhibiting the mycelial growth of seven pathogenic fungi
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com