Diethoxy thiophosphate organophosphorus pesticide antibody, and preparation and use thereof
A technology of diethoxyphosphorothioate and ethoxyphosphorothioate, which is applied in the field of immunology, can solve the problems that pesticides are not targeted, not solved well, and the detection limit is very different. The method is simple and convenient, Low detection cost, good stability and repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
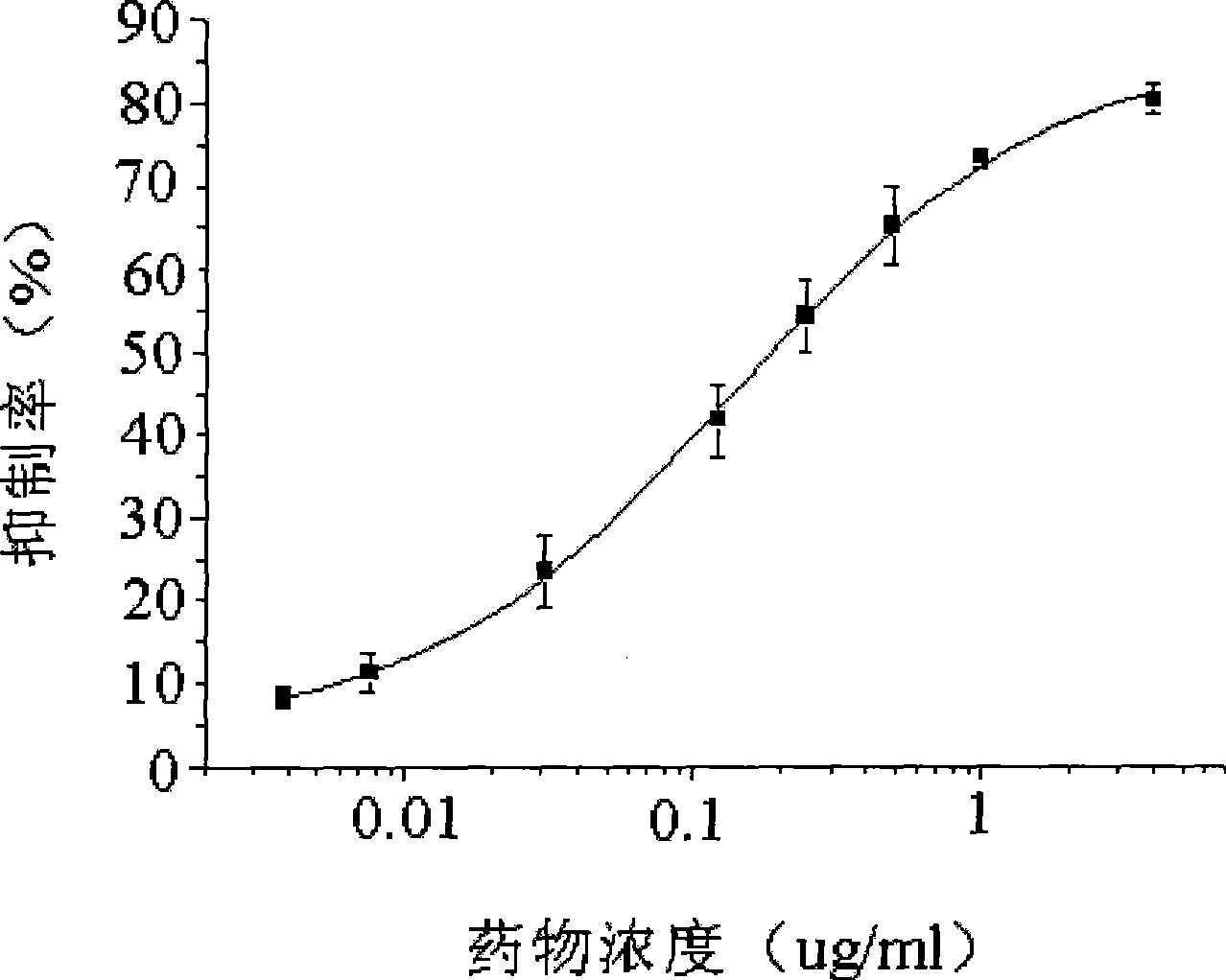
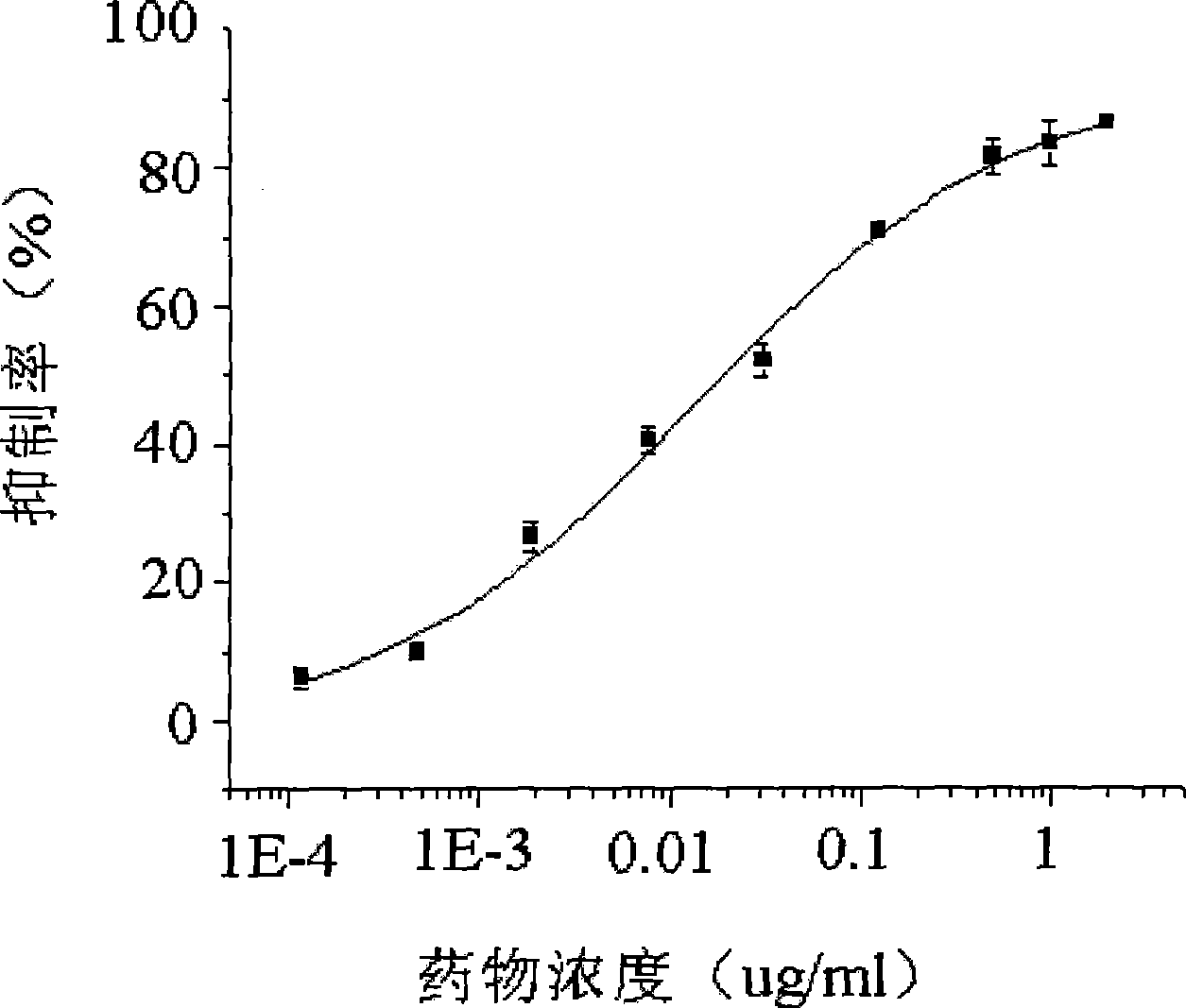
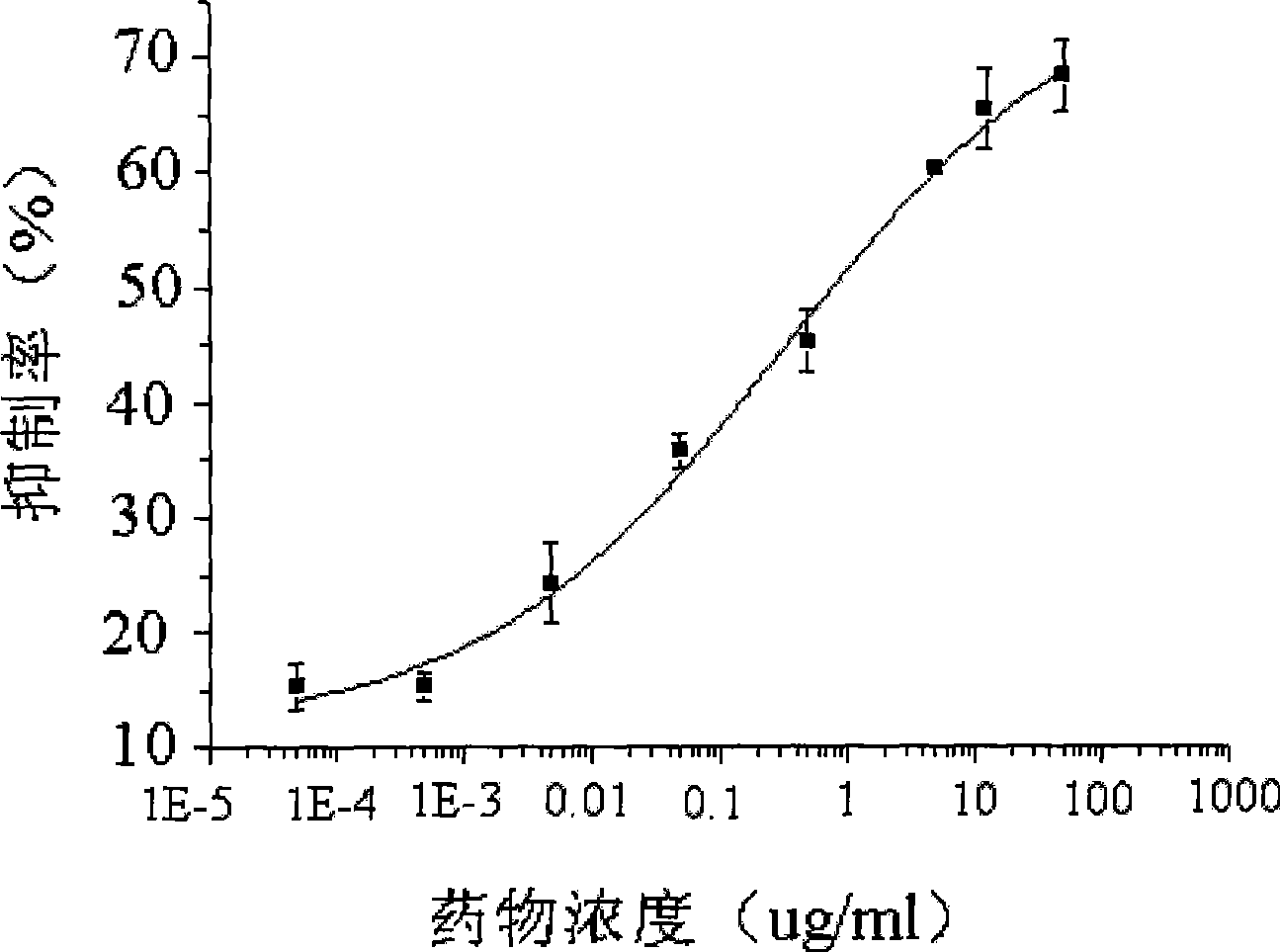
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of Diethoxyphosphorothioate Organophosphorus Pesticide Immune Hapten OPH1
[0052]Take 4.88g (0.04moL) of p-hydroxybenzaldehyde and dissolve it in pyridine, protect it with a drying tube, put it in a water bath at 85°C, and stir. Add 3.75 g (0.036 moL) of malonic acid dropwise to the above solution and react for 3 h. After the reaction was completed, the mixture was poured into a saturated sodium bicarbonate solution, stirred for 15 min, extracted with ethyl acetate, and the pH of the aqueous layer was adjusted to 3 with 4M hydrochloric acid, and 2.32 g of a white solid was precipitated, with a yield of 78.6%. ESI-MS analysis (negative) m / z 163[M-H]-; 1H-NMR (400MHz, d6-Acetone and TMS): δ 6.33 (d, J=16.0Hz, 1H, CH=CH); 6.89 (d, J=8.6Hz, 2H, ArH); 7.54(d, J=8.6Hz, 2H, ArH); 7.60(d, J=16.0Hz, 1H, CH=CH); 8.86(s, 1H, OH); 10.77 (s, 1H, COOH).
[0053] Weigh 0.7 (0.0125 moL) KOH and dissolve it in anhydrous methanol, weigh 0.821 g (0.005 moL) of th...
Embodiment 2
[0056] Example 2 Preparation of Diethoxyphosphorothioate Organophosphorus Pesticide Coating Hapten OPH2
[0057] Weigh 1.68g (0.018moL) KOH and dissolve in anhydrous methanol, weigh 1.00g (0.01moL) aminobutyric acid, dissolve in the above solution with stirring at 0°C, and stir for 30min. Add 1.5g (0.008moL) diethoxyphosphoryl thiochloride dropwise, and react at 0°C for 12h. After the reaction, spin dry, wash with chloroform, filter, and the supernatant is concentrated and passed through a (chloroform:methanol=3:1) silica gel column to obtain 1.14g, with a yield of 56.0%. ESI-MS analysis (negative) m / z 254[M-H]-; 1H-NMR (400MHz, CDCl 3 and TMS): δ 1.27 (t, J=7.0Hz, 6H, CH 3 ); 1.77 (p, J=6.9Hz, 2H, CH 2 ); 2.39(t, J=7.3Hz, 2H, CH 2 ); 2.98 (td, J 1 =6.8Hz,J 2 =6.8Hz,J 3 =6.8Hz,J 4 =11.1Hz, 2H, CH 2 ); 4.01 (ddd, J 1 = 1.4Hz,J 2 =7.3Hz,J 3 =15.9Hz, 4H, CH 2 ).
[0058] The prepared coated hapten (OPH2) structure is shown in formula (II):
[0059]
Embodiment 3
[0060] Example 3 Preparation of Diethoxyphosphorothioate Organophosphorus Pesticide Immune Antigen OPH1-BSA
[0061] (1) Dissolve 0.12mmol of hapten in 2ml of DMF, stir and add DCC and NHS. Magnetic stirring was carried out overnight at 4°C, and the supernatant was liquid A after centrifugation;
[0062] (2) Weigh 0.002mol BSA, slowly dissolve it in 10ml of PBS (pH8.0) with a concentration of 0.1mol / L at 4°C, stir and dissolve to prepare solution B;
[0063] (3) Under magnetic stirring, liquid A was gradually dropped into liquid B, and reacted at 4°C for 12 hours. After centrifugation, the supernatant was taken, dialyzed with normal saline at 4°C for 3 days, dispensed into 0.5ml centrifuge tubes at a concentration of 1 mg / mL or 2 mg / mL, and stored in a -20°C refrigerator for later use.
[0064] Immunogen identification: The carrier protein BSA, hapten and immunogen were detected by UV scanning (200-400nm), and it was found that the absorption curve of the immunogen OPH1-BSA ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com