Preparation method of ofloxacin sustained-release capsules
A technology of ofloxacin and sustained-release capsules is applied in the field of ofloxacin sustained-release capsule preparations to achieve the effects of slowing absorption, smoothing the shape of the time curve, and avoiding sudden release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
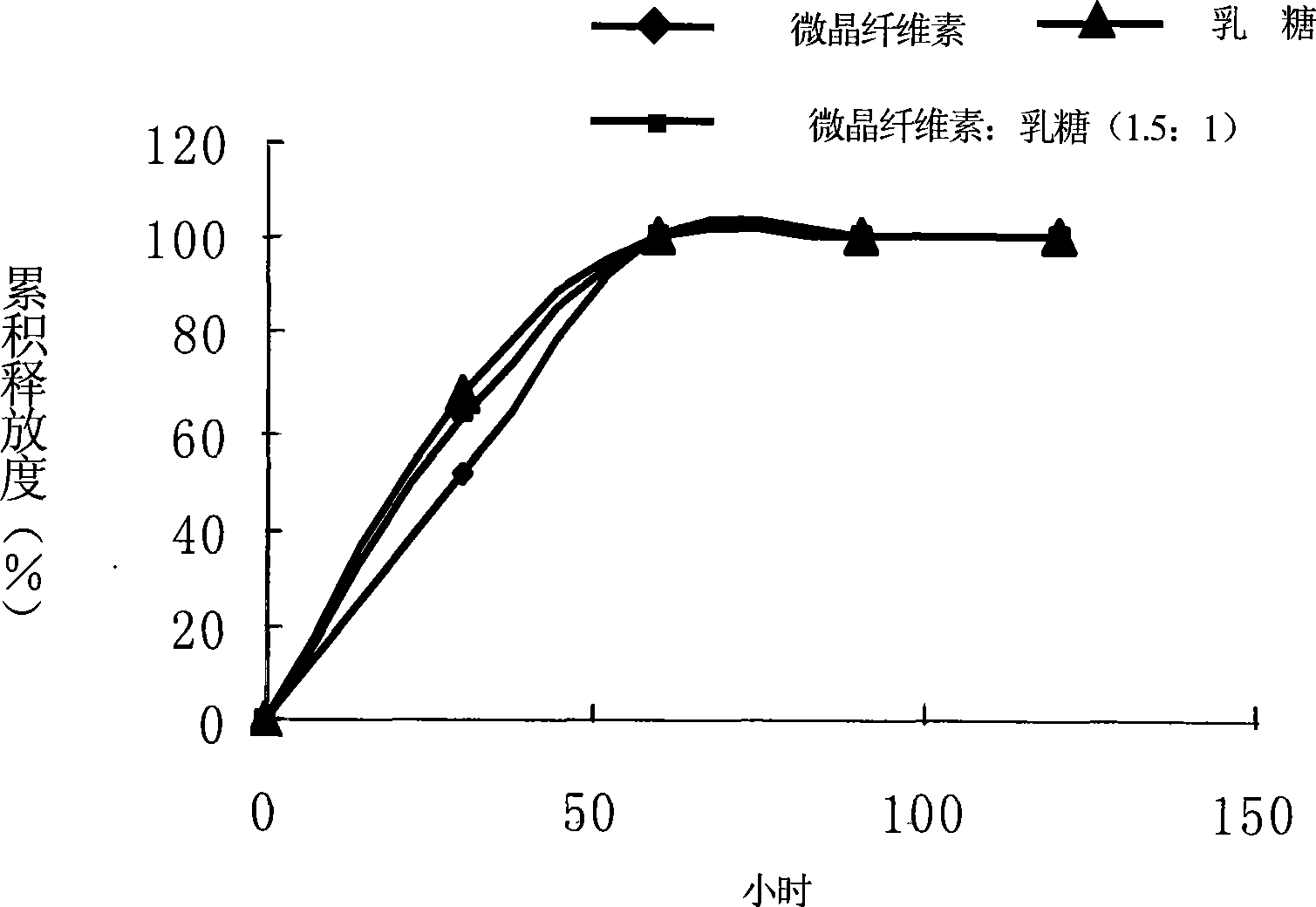
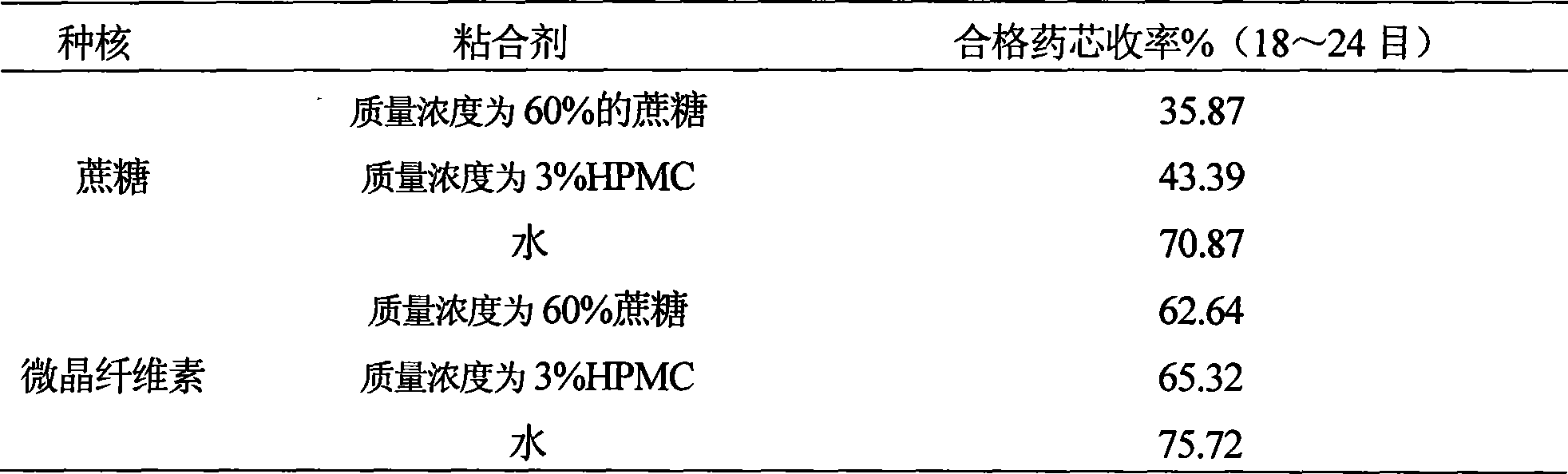
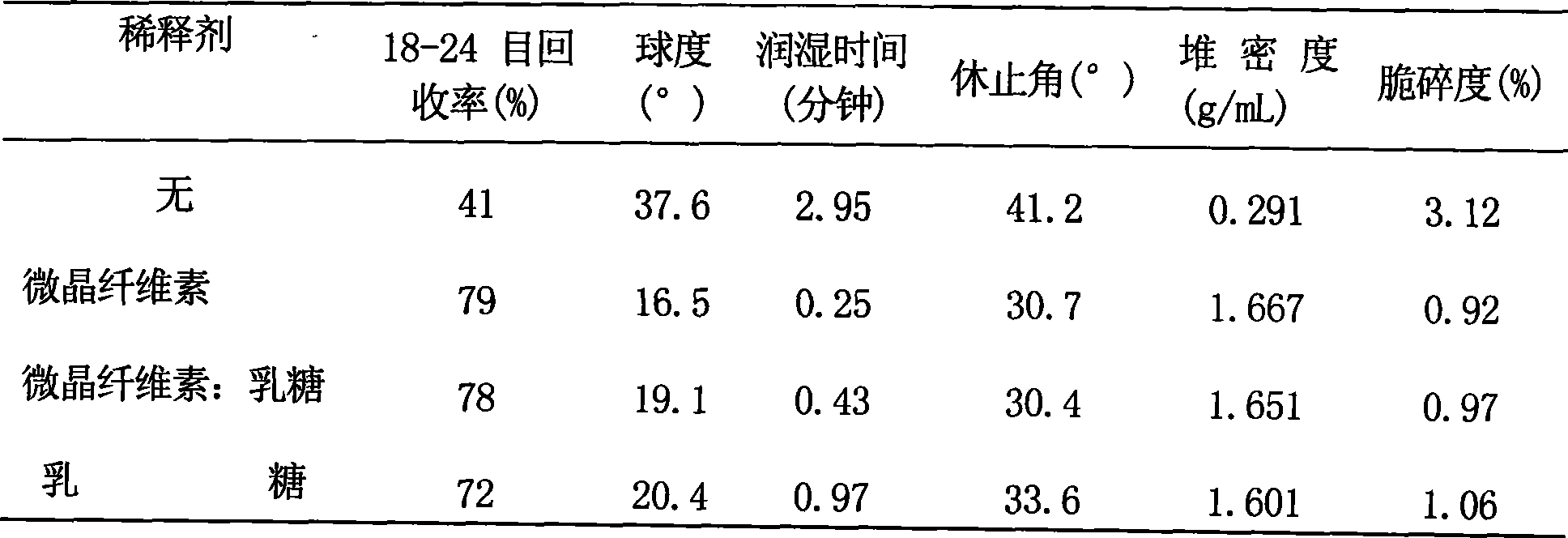
Image
Examples
Embodiment 1
[0048] Taking the production of 1000 ofloxacin sustained-release capsule products as an example, its preparation method is as follows:
[0049] 1. Ingredients
[0050] Weigh the medicinal active ingredients and auxiliary materials according to the following mass ratios:
[0051] Ofloxacin 200g
[0052] Microcrystalline Cellulose 42.43g
[0053] Lactose 28.28g
[0054] Citric acid 15g
[0055] The weight proportion of the used raw materials of above-mentioned medicine is:
[0056] Ofloxacin 70.00%
[0057] Microcrystalline Cellulose 14.85%
[0058] Lactose 9.90%
[0059] Citric acid 5.25%
[0060] 2. mix
[0061] Grind ofloxacin through a 120-mesh sieve, crush lactose through an 80-mesh sieve, and citric acid through an 80-mesh sieve, weigh ciprofloxacin hydrochloride, lactose, and citric acid according to the mass ratio and mix them uniformly with a mixer to prepare into a mixture.
[0062] 3. Prepare vegetarian pills
[0063] Grind the microcrystalline cellulose t...
Embodiment 2
[0085] Taking the production of 1000 ofloxacin sustained-release capsule products as an example, its preparation method is as follows:
[0086] In batching step 1 of the present invention, the consumption of used ofloxacin, the consumption of auxiliary material are identical with embodiment 1.
[0087] In step 3 of preparing the plain pill of the present invention, the polishing time is 2 minutes, and other steps are the same as in Example 1.
[0088] In the coating step 4 of the present invention, when the coating amount of Suwan 1000g is 4.0%, the coating solution is made from the raw materials of the following mass ratio:
[0089] Aqueous dispersion of ethyl acrylate-methyl methacrylate copolymer 10.98g
[0090] Aqueous dispersion of methacrylic acid-ethyl acrylate copolymer 87.50g
[0092] Polyethylene glycol 6000 5.49g
[0093] Sodium Lauryl Sulfate 0.55g
[0094] Water 449.05mL
[0095] The mass percent of above-mentioned coating solution...
Embodiment 3
[0104] Taking the production of 1000 ofloxacin sustained-release capsule products as an example, its preparation method is as follows:
[0105] In batching step 1 of the present invention, the consumption of used ofloxacin, the consumption of auxiliary material are identical with embodiment 1.
[0106] In step 3 of preparing the plain pill of the present invention, the polishing time is 8 minutes, and other steps are the same as in Example 1.
[0107] In the coating step 4 of the present invention, when the coating amount of Suwan 1000g is 4.0%, the coating solution is made from the raw materials of the following mass ratio:
[0108] Aqueous dispersion of ethyl acrylate-methyl methacrylate copolymer 22.36g
[0109] Aqueous dispersion of methacrylic acid-ethyl acrylate copolymer 175.70g
[0111] Polyethylene glycol 6000 10.98g
[0112] Sodium Lauryl Sulfate 0.11g
[0113] Water 328.93mL
[0114] The mass percent of above-mentioned coating solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com