Method for preparing pyrogallic acid with glyoxaline as gallic acid decarboxylation catalyst
A technology of pyrogallic acid and gallic acid, which is applied in chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve the problems of high cost and complicated process, and achieve low cost, simple process and high reaction rate. The effect of low temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
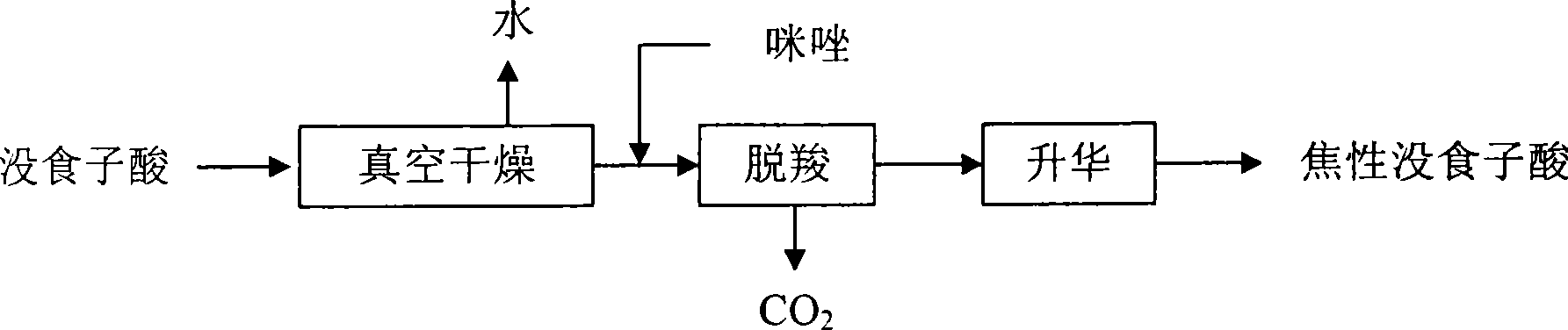
[0015] Weigh 200g of gallic acid raw material with 1 molecule of crystal water, place it in a petri dish, and dry it for 3 hours at a system pressure of 4.0kPa and a temperature of 100°C. The amount of dried material is 181.1g. Weigh 30.0 g (0.176 mol) of dried gallic acid, add it into a 100-mL three-necked flask equipped with a stirrer, a thermometer and a condenser, add 3.0 g of imidazole (purity is 99.0%), and gradually heat to 145 ~150°C, the color of the material gradually changes from white to gray, and then to dark gray, and the material gradually changes from solid powder to molten, and a large amount of CO2 gas escapes at the same time. Insulation reaction until no bubble escapes, sampling and detection by TLC (silica gel plate chromatography, 10% FeCl 3 The aqueous solution is used as the developer, and the developer is V (acetone): V (60-90°C petroleum ether) = 1.5: 1, add 2-3 drops of glacial acetic acid to eliminate the tailing phenomenon). Vacuumize the reaction...
Embodiment 2
[0017] Weigh 100.0 g (0.587 mol) of dried gallic acid, add it into a 250-mL three-necked flask equipped with a stirrer, a thermometer and a condenser, add 8.0 g of imidazole (purity is 99.0%), and gradually heat to 145 ~150°C, the color of the material gradually changes from white to gray, and then to dark gray, and the material gradually changes from solid powder to molten, and there is a large amount of CO 2 Gas escapes. Insulate the reaction until no bubbles escape, take a sample and use TLC detection (silica gel plate chromatography, 10% FeCl3 aqueous solution as the color developer, the developer is V (acetone): V (60 ~ 90 ° C petroleum ether) = 1.5: 1 , add 2 to 3 drops of glacial acetic acid to eliminate tailing phenomenon). Vacuumize the reaction system until the pressure of the system is 2.67kPa, and gradually raise the temperature to 160°C. At this time, a white sublimation product, pyrogallic acid, escapes. Keep this temperature until no sublimation product escapes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com