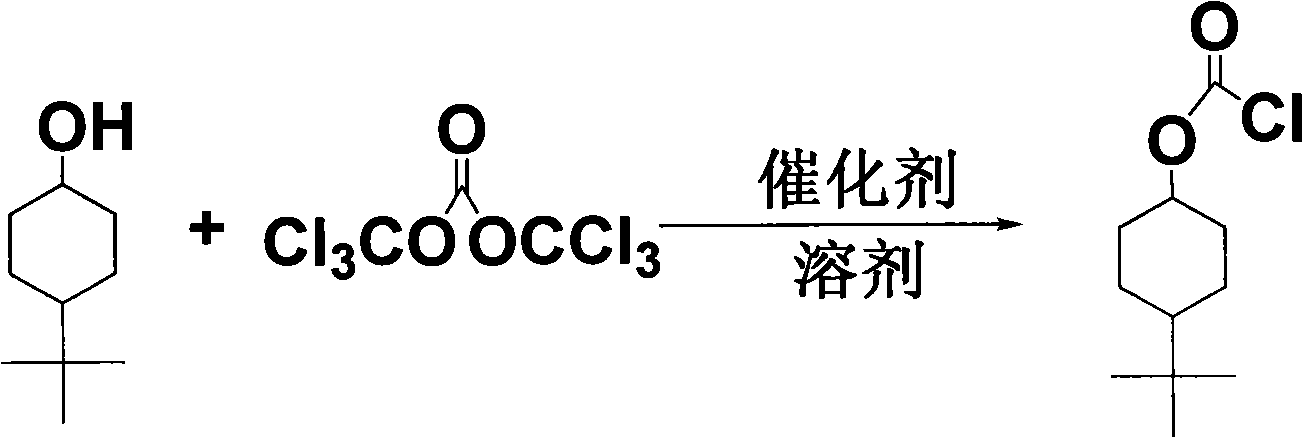
Synthesis of 4-tert-butyl cyclohexyl chloride formic ester
A technology of tert-butyl cyclohexanol and synthesis method, which is applied in the chemical synthesis of 4-tert-butyl cyclohexyl chloroformate and the field of synthesizing 4-tert-butyl cyclohexyl chloroformate, can solve the problem that the catalyst is not easy to recover, the post-processing is complicated and safe hidden dangers and other problems, to achieve the effect of good practical value, great economic value, and pollution reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 250ml round bottom flask, add 150ml of methylene chloride; under a magnetic stirrer, 4.6881g (30mmol) of 4-tert-butylcyclohexanol and 6.2530g (21mmol) of bis(trichloromethyl)carbonate are added to In a round-bottomed flask, stir to make it fully dissolved; add 4g of artificial zeolite, and stir and react at room temperature for 24h. After the reaction was completed, the catalyst was filtered out, and the mother liquor was rotary distilled to evaporate the solvent. After mother liquor is concentrated again, pass column separation (the solvent that column separation adopts is sherwood oil and methylene dichloride, and the volume ratio of sherwood oil and methylene chloride is 2: 1), finally the product that separates is added appropriate sherwood oil, in Recrystallization was carried out in the freezer for 24 hours to obtain white crystals. It was filtered and dried to obtain 5.9 g of white 4-tert-butylcyclohexyl chloroformate crystals, with a yield of 90%. After t...
Embodiment 2
[0021] In a 250ml round bottom flask, add 50ml of dichloromethane; under a magnetic stirrer, add 2.3440g (15mmol) of 4-tert-butylcyclohexanol and 3.1265g (10.5mmol) of bis(trichloromethyl)carbonate Put it into a round bottom flask, stir to make it fully dissolved; add 2g of Y-type molecular sieves. Other preparation conditions and steps were the same as in Example 1, and 2.6 g of white 4-tert-butylcyclohexyl chloroformate crystals were obtained with a yield of 79% and a purity of 98% after testing. After FTIR analysis, at 1799cm -1 、1237cm -1 、1081cm -1 There are obvious characteristic peaks indicating that it is consistent with the target product.
Embodiment 3
[0023] In a 250ml round bottom flask, add 50ml of dichloromethane; under a magnetic stirrer, add 2.3440g (15mmol) of 4-tert-butylcyclohexanol and 3.1265g (10.5mmol) of bis(trichloromethyl)carbonate Put it into a round bottom flask, stir to make it fully dissolved; add 2g of activated carbon. Other preparation steps and conditions were the same as in Example 1 to obtain 2.6 g of white 4-tert-butylcyclohexyl chloroformate crystals with a yield of 79%. After testing, the purity is 99%. After FTIR analysis, at 1799cm -1 、1237cm -1 、1081cm -1 There are obvious characteristic peaks indicating that it is consistent with the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com