Method for synthesis of aniline and alkali-chloride with electrochemical conjugate synthesis
An electrochemical and electrolytic method, applied in the field of electrochemical synthesis of chlor-alkali and paired aniline, can solve the problems of precious metal loss, poor anti-toxicity, high production cost, etc., and achieve the effect of saving consumption and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
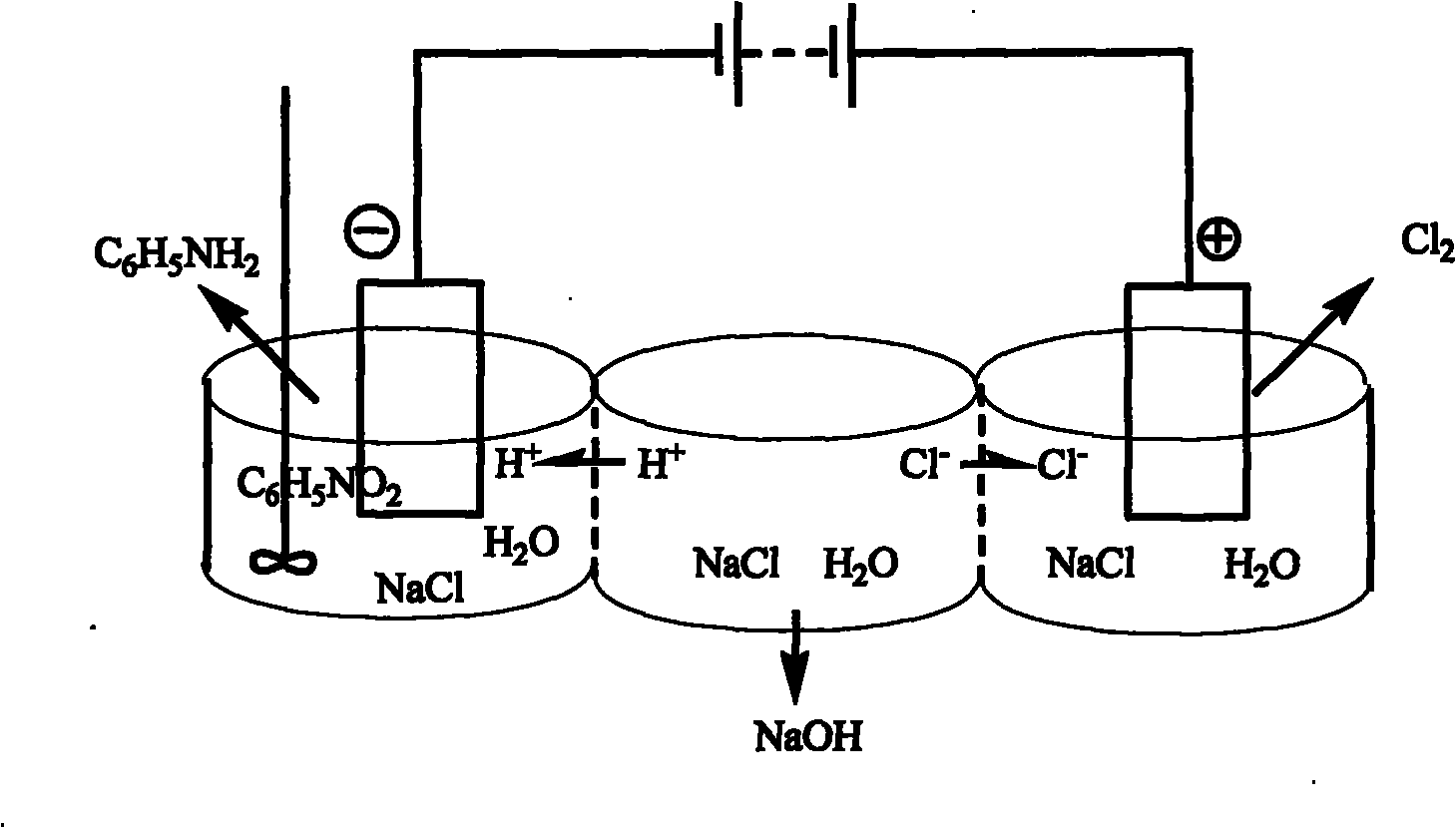
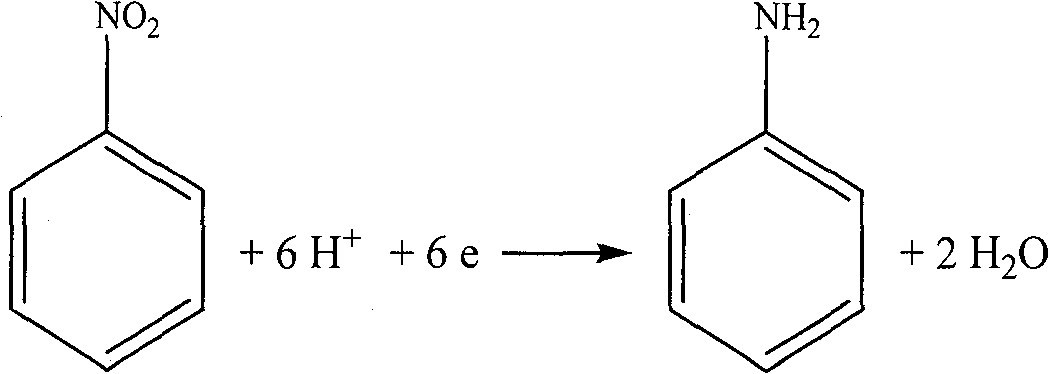
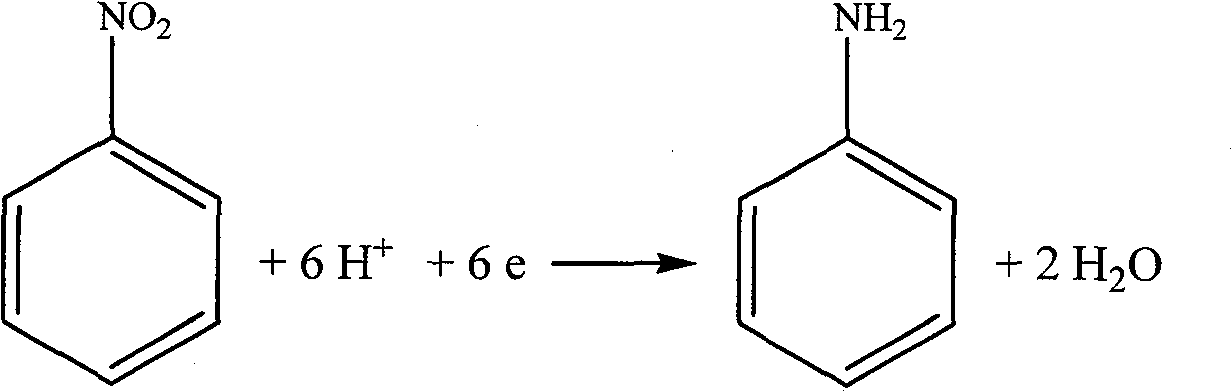
[0041] In a three-chamber electrolytic cell of 250 mL in each chamber, NaCl solution with a concentration of 300 g / L is added to the anode chamber and the middle chamber, and 5% nitrobenzene and 0.5% triethylhexadecyl ammonium bromide are added to the cathode chamber , 20% sodium chloride, 1% hydrochloric acid. The anode is graphite and the cathode is copper. The cation exchange membrane is a PE-100 cation exchange membrane, and the anion exchange membrane is a polyethylene anion exchange membrane. Apply direct current between the cathode and anode for electrolysis. The chlorine gas produced is collected at the anode. After the reaction was completed, the catholyte was extracted with ether to separate aniline. The middle chamber separates sodium hydroxide through processes such as concentration. The anode current efficiency is greater than 85%, and the cathode current efficiency is greater than 85%.
Embodiment 2
[0043] In each chamber is a 250mL three-chamber electrolytic cell, the anode chamber and the middle chamber are all added with a concentration of 300g / L NaCl solution, and the cathode chamber is added with 5% nitrobenzene, 0.5% sodium dodecylbenzenesulfonate, 20 % sodium chloride, 1% hydrochloric acid. The anode is DSA, and the cathode is stainless steel sheet. The cation exchange membrane is a PE-100 cation exchange membrane, and the anion exchange membrane is a polyethylene anion exchange membrane. Apply direct current between the cathode and anode for electrolysis. The chlorine gas produced is collected at the anode. After the reaction was completed, the catholyte was extracted with ether to separate aniline. The middle chamber separates sodium hydroxide through processes such as concentration. The anode current efficiency is greater than 92%, and the cathode current efficiency is greater than 85%.
Embodiment 3
[0045] In each chamber is 250mL three-chamber electrolytic cell, the anode chamber and the middle chamber are added with a concentration of 300g / L NaCl solution, and the cathode chamber is added with 5% nitrobenzene, 0.5% OP-10, 20% sodium chloride, 1% hydrochloric acid. The anode is DSA and the cathode is graphite. The cation exchange membrane is perfluorosulfonic acid type cation exchange membrane Nafion901, and the anion exchange membrane is polyethylene type anion exchange membrane. Apply direct current between the cathode and anode for electrolysis. The chlorine gas produced is collected at the anode. After the reaction was completed, the catholyte was extracted with ether to separate aniline. The middle chamber separates sodium hydroxide through processes such as concentration. The anode current efficiency is greater than 92%, and the cathode current efficiency is greater than 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com