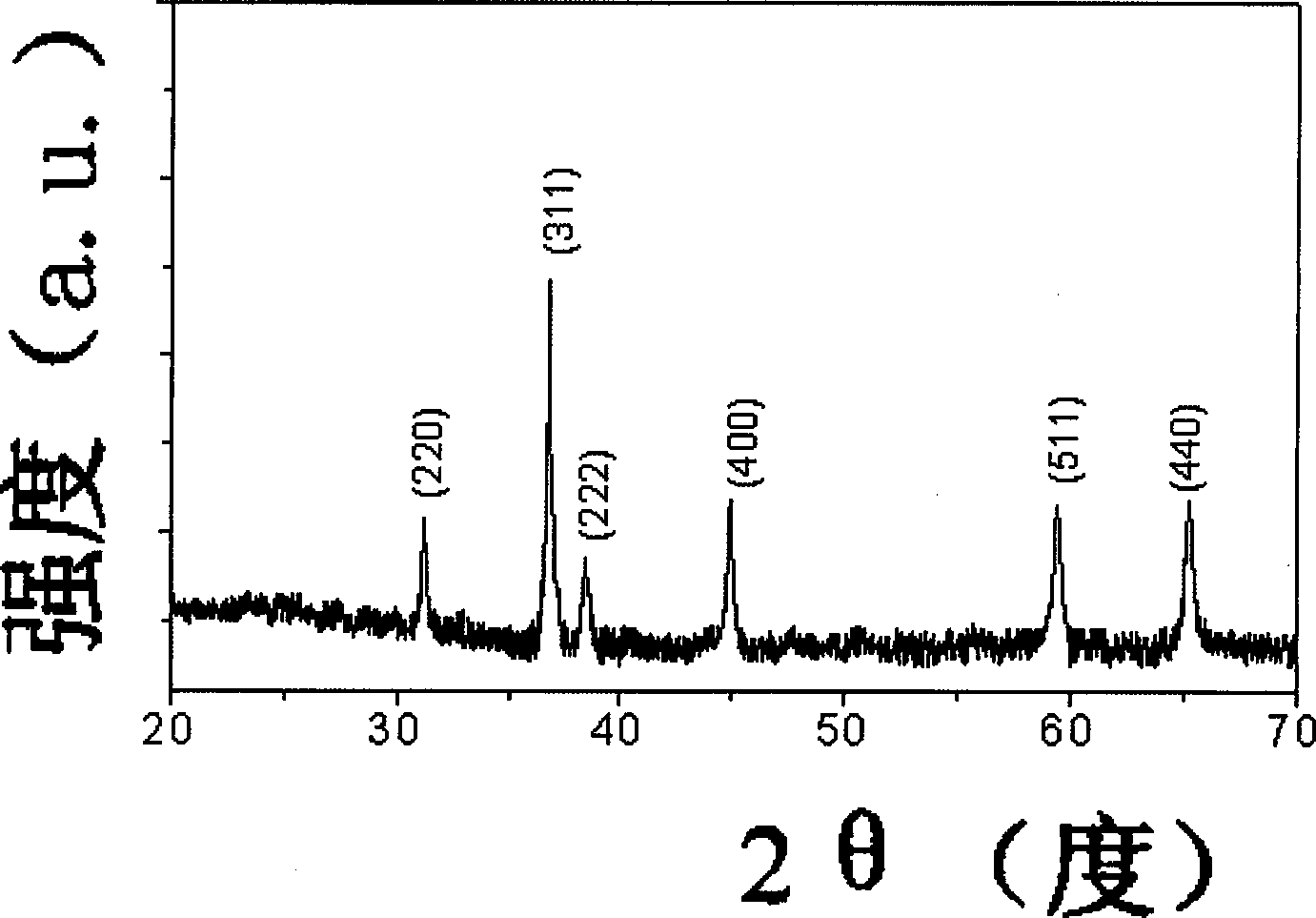
Process for producing stephanoporate one-dimensional nano-cobaltic-cobaltous oxide
A technology of tricobalt tetroxide and nanometer, applied in the direction of cobalt oxide/cobalt hydroxide, electrical components, battery electrodes, etc., can solve the problems of difficult to meet the requirements of battery performance improvement, uneven distribution of product particles, high temperature and equipment requirements, etc. Achieve the effects of low cost, high bulk density and short production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Weigh 1mmol cobalt chloride (CoCl 2 ), was added to 25 mL of distilled water, and 1 mmol of urea was added under magnetic stirring to form a mixed solution. Then the mixed solution was heated at 80°C, and the reaction time was controlled for 1 hour. Then filter, wash, and dry in a 70°C drying oven for 30 minutes. Then calcined at 200° C. for 1 hour to obtain multifunctional cobalt tetraoxide nanowires with a purity of 100%, a particle size of 40 nm, and a yield of 87.2% by weight.
Embodiment 2
[0029] Weigh 1 mmol of cobalt chloride under vigorous stirring, add it into 25 mL of distilled water, and continue adding 1 mmol of urea under magnetic stirring to form a mixed solution. Then the mixed solution was heated at 100°C, and the reaction time was controlled for 1 hour. After filtering, washing, and drying in a drying oven at 70° C. for 5 hours. Then calcined at 300° C. for 1.5 hours to obtain multifunctional cobalt tetroxide nanorod clusters with a purity of 100%, a particle size of 50 nm, and a yield of 87.1% by weight.
Embodiment 3
[0031] Weigh 1 mmol of cobalt chloride under vigorous stirring, add it into 25 mL of distilled water, and continue adding 1 mmol of urea under magnetic stirring to form a mixed solution. Then the mixed solution was heated at 120°C, and the reaction time was controlled for 1 hour. Then filter, wash, and dry in a 70°C drying oven for 12 hours. Then calcined at 400° C. for 2 hours to obtain dispersed multifunctional cobalt tetroxide nanorods with a purity of 100%, a particle diameter of 50 nm, and a yield of 86.9% by weight.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com