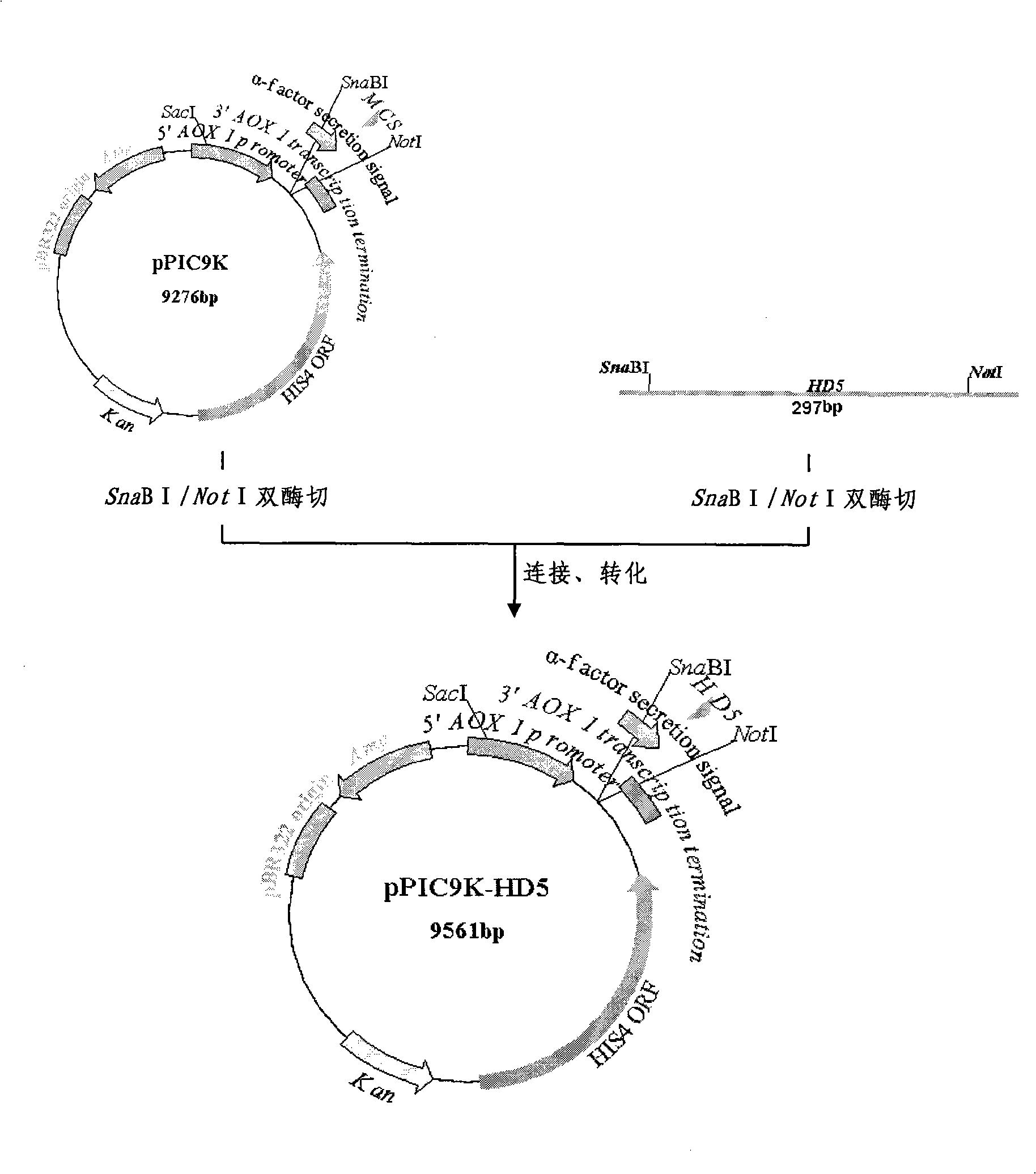
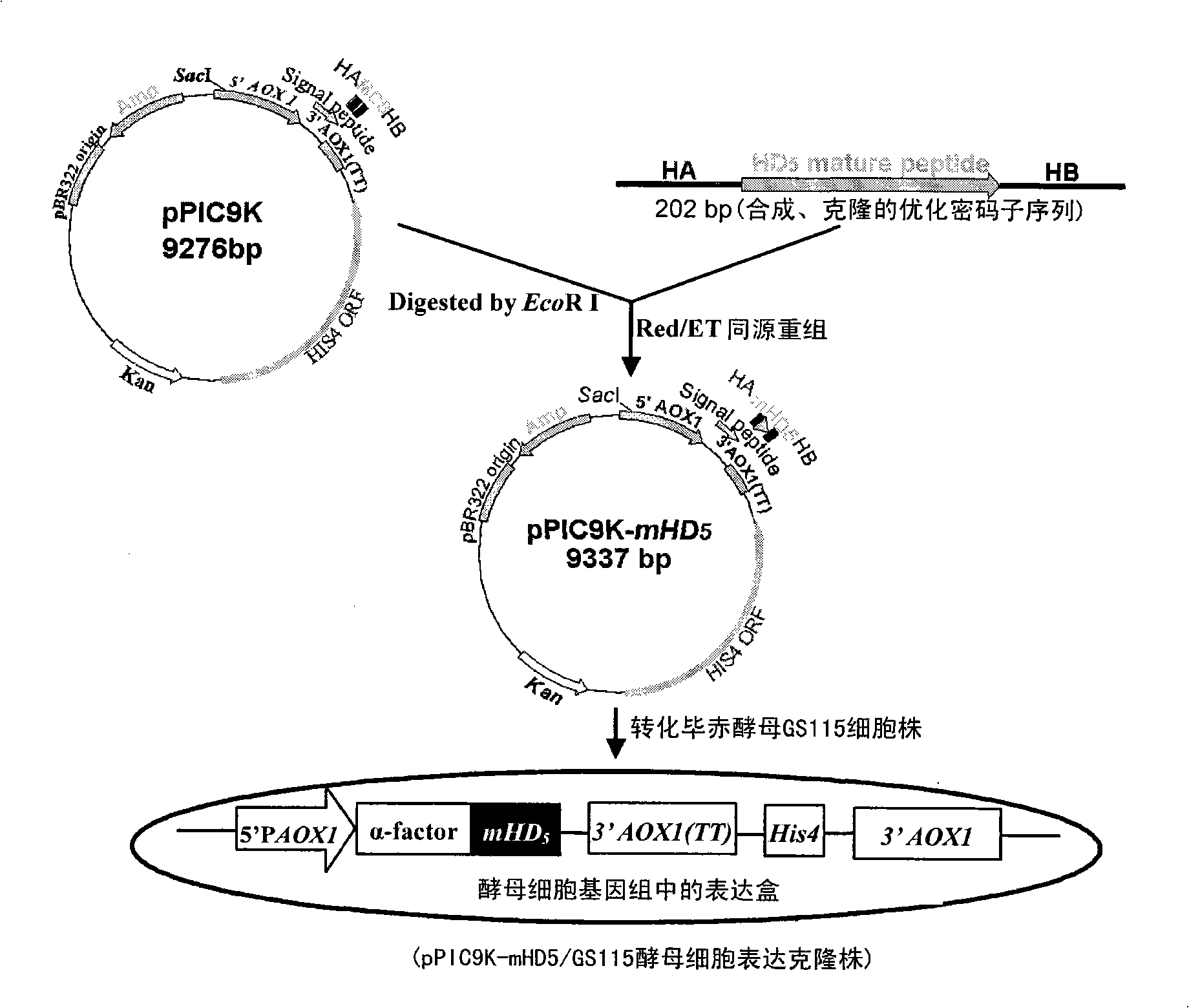
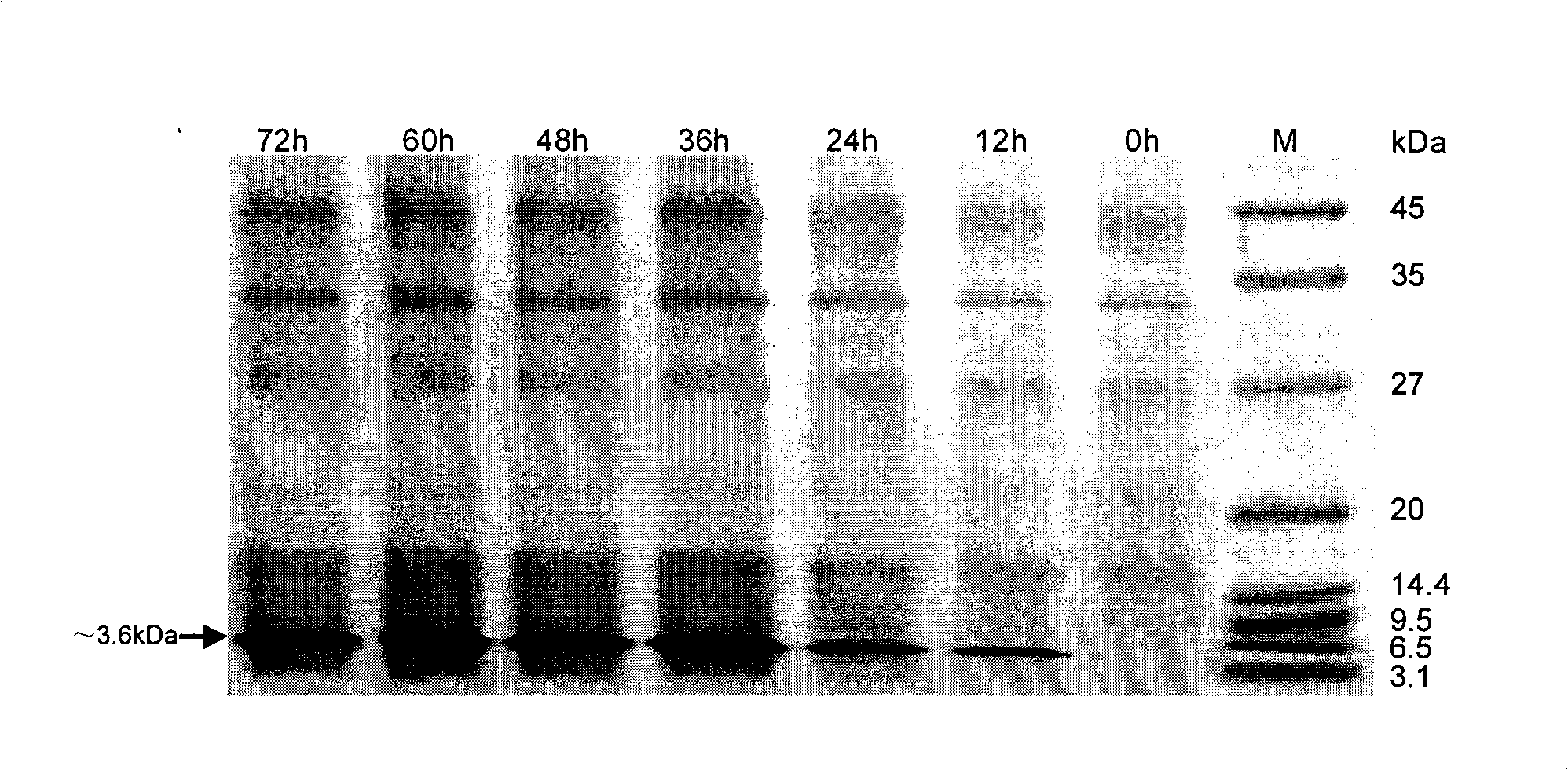
Gene engineering preparation method of bioactive peptide containing human alpha defensin 5
A genetic engineering and defensin technology, applied in the field of bioengineering pharmacy, can solve problems such as the preparation process that has not yet been established
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1 Cloning of human α-defensin 5 gene (HD5)
[0114] Obtain the cDNA sequence of HD5 gene by RT-PCR method from the ileal mucosa sample of patients with common enteritis (the sample in this example is from the First Affiliated Hospital of the Third Military Medical University of the Chinese People's Liberation Army), and the PCR upstream and downstream primers used for amplification are respectively HD -sense01 and HD-antisense01. The base sequences of the primers are as follows (SEQ ID NO: 3-4 in sequence):
[0115] HD-sense01: 5'-CGT AAG CTT ATA TCC ACT CCT GCT CTC CCT-3'
[0116] HD-antisense01: 5'-ATC GCG GCC GCA ATG CTT GAA CTT TAT TTT G-3'
[0117] Refer to the instructions of the RT-PCR kit (One Step RNA PCR Kit) to establish the RT-PCR reaction system as follows: 5.0 μl 10 mM dNTP, 10.0 μl 25 mM MgCl 2 , 1.0μl RNase Inhibitor (40U / μl), 2.0μl each primer (20mmol / L), 2.0μl RNA, 1.0μl AMV RtaseXL (5U / μl), 1.0μl Taq enzyme, 5.0μl 10× buffer, 21.0μl ddH ...
Embodiment 2
[0117] Refer to the instructions of the RT-PCR kit (One Step RNA PCR Kit) to establish the RT-PCR reaction system as follows: 5.0 μl 10 mM dNTP, 10.0 μl 25 mM MgCl 2 , 1.0μl RNase Inhibitor (40U / μl), 2.0μl each primer (20mmol / L), 2.0μl RNA, 1.0μl AMV RtaseXL (5U / μl), 1.0μl Taq enzyme, 5.0μl 10× buffer, 21.0μl ddH 2O. Then follow the following procedures: ①reverse transcription: 50°C, 30 minutes; ②denaturation: 94°C, 2 minutes; ③denaturation: 94°C, 30 seconds; ④refolding: 55°C, 30 seconds; ⑤extension: 72°C , 45 seconds; ⑥Return to step "③", 34 cycles; ⑦Extend: 72°C, 7 minutes; ⑧Save at 4°C, the total number of cycles is 32 times. The obtained RT-PCR product was subjected to 1% agarose gel electrophoresis (80V, electrophoresis for 30 minutes), and the result showed that a DNA band with a size of about 450bp was amplified. After the PCR product was purified and recovered, it was ligated with the pMD18-T vector. The ligation product was transformed into DH5α Escherichia coli co...
Embodiment 3
[0139] Example 3 Fermentation of Yeast Engineering Strain and Purification of Recombinant Human α-Defensin 5 Active Peptide
[0140] Inoculate pPIC9K-mHD5-integrated yeast engineered strains frozen at -70°C on YPD plates for overnight culture and activation at 30°C, select a single colony with a good appearance and inoculate it in BMGY medium, and culture in a constant temperature shaker at 28°C and 30°C at 220rpm for 16 ~18h to OD 600 ≈3~5, then transfer to OD once at 1:10 600 It is about 4, and the bacterial liquid is used as the seed liquid. Then the seed solution is transplanted into the basal salt medium (adding trace salt PTM 1 ) in a 15L fermenter (B.Braun company, Germany) for high-density culture and fermentation. The fermentation temperature was controlled at 30°C (28°C after methanol induction), the dissolved oxygen content was controlled between 30% and 40%, the pH value was controlled at 5.0 (3.5 after methanol induction), and the cells were cultured to OD 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com