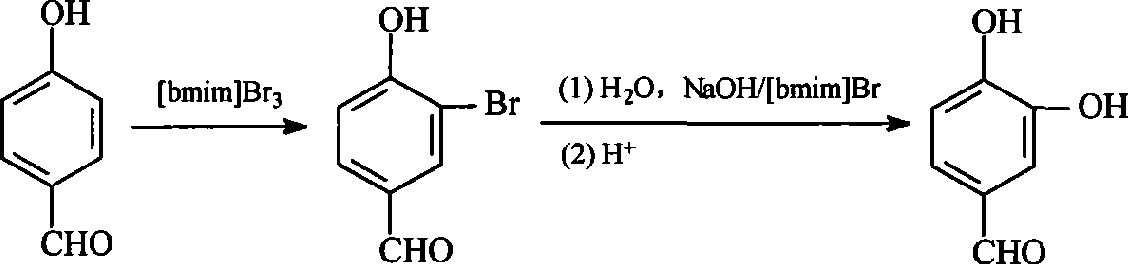
A method for preparing 3, 4-dihydroxy benzaldehyde in ionic liquid with one-pot method
A technology of dihydroxybenzaldehyde and p-hydroxybenzaldehyde is applied in the field of manufacture of dihydroxybenzaldehyde compounds, can solve the problems of long cyanide or process route, unfavorable industrialized production, and high raw material price, and achieves short process route and high production efficiency. Low cost and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Add 0.02mol of p-hydroxybenzaldehyde and 0.02mol of ionic liquid 1-butyl-3-methylimidazole tribromide to a three-necked flask, and carry out bromination reaction at 20°C for 30 minutes under stirring;
[0028] (2) 10mL of sodium hydroxide aqueous solution that sodium hydroxide and p-hydroxybenzaldehyde mol ratio are 1.25: 1 is added in the reaction solution that step (1) obtains, heat up to 100 ℃ under stirring state, and at this temperature Under hydrolysis reaction for 2 hours;
[0029] (3) The reaction solution obtained in step (2) was mixed with 0.1mol L -1 The hydrochloric acid solution was acidified to pH 5, then extracted with ethyl acetate, the extract was washed with saturated brine to pH 7, dried and evaporated to remove ethyl acetate, and the obtained substance was recrystallized with water to obtain 3,4-dihydroxybenzaldehyde white crystals.
[0030] (4) The remaining liquid after the extraction of step (3) was washed twice with 20mL ethyl acetate, and ...
Embodiment 2
[0033] (1) Add 0.02mol p-hydroxybenzaldehyde and 0.021mol ionic liquid 1-butyl-3-methylimidazole tribromide to a three-necked flask, and carry out bromination reaction at 25° C. for 25 minutes under stirring;
[0034] (2) 10mL of aqueous sodium hydroxide solution with a 1.5:1 mol ratio of sodium hydroxide and p-hydroxybenzaldehyde was added to the reaction solution obtained in step (1), heated to 120° C. under stirring, and at this temperature Lower hydrolysis reaction 1.5 hours;
[0035] (3) The reaction solution obtained in step (2) was mixed with 0.1mol L -1 The hydrochloric acid solution was acidified to pH 5, then extracted with ethyl acetate, the extract was washed with saturated brine to pH 7, dried and evaporated to remove ethyl acetate, and the obtained substance was recrystallized with water to obtain 3,4-dihydroxybenzaldehyde white crystals.
[0036] The obtained 3,4-dihydroxybenzaldehyde white crystals were 2.08 g, the yield was 75.2%, the melting point was 152-1...
Embodiment 3
[0039] (1) Add 0.02mol p-hydroxybenzaldehyde and 0.022mol ionic liquid 1-butyl-3-methylimidazole tribromide to a three-necked flask, and carry out bromination reaction at 15°C for 35 minutes under stirring;
[0040] (2) Add 10 mL of aqueous sodium hydroxide solution with a mol ratio of sodium hydroxide to p-hydroxybenzaldehyde of 1:1 to the reaction solution obtained in step (1), heat up to 80° C. under stirring, and Hydrolysis reaction for 2.5 hours;
[0041] (3) The reaction solution obtained in step (2) was mixed with 0.1mol L -1 The hydrochloric acid solution was acidified to pH 5, then extracted with ethyl acetate, the extract was washed with saturated brine to pH 7, dried and evaporated to remove ethyl acetate, and the obtained substance was recrystallized with water to obtain 3,4-dihydroxybenzaldehyde white crystals.
[0042] 1.81 g of white crystals of 3,4-dihydroxybenzaldehyde were obtained, the yield was 65.6%, the melting point was 152-153° C., and the purity was 99...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com