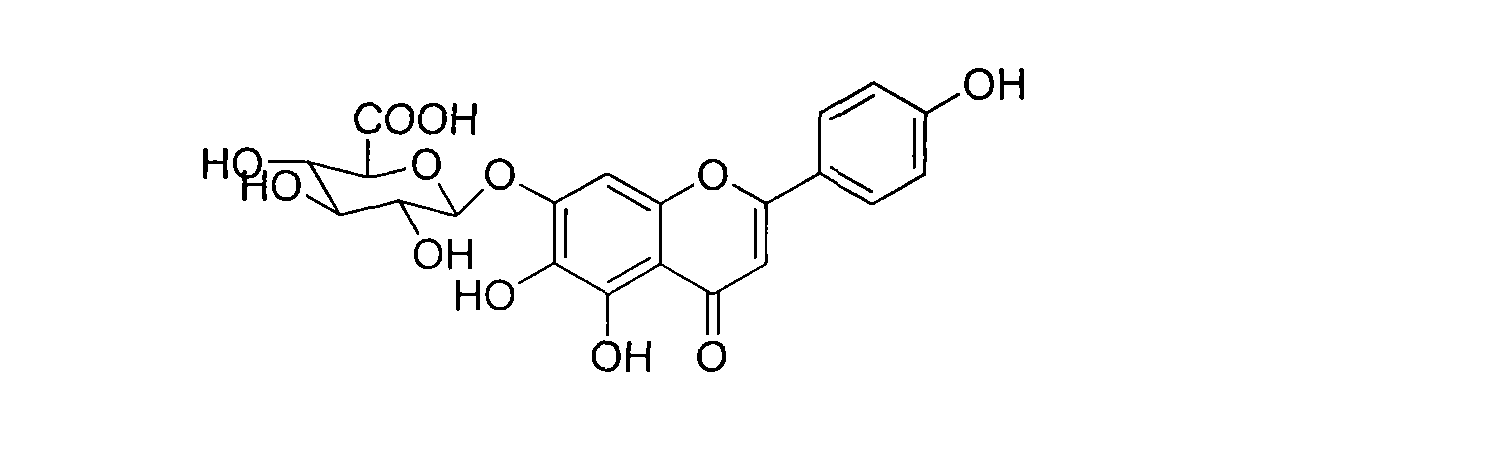
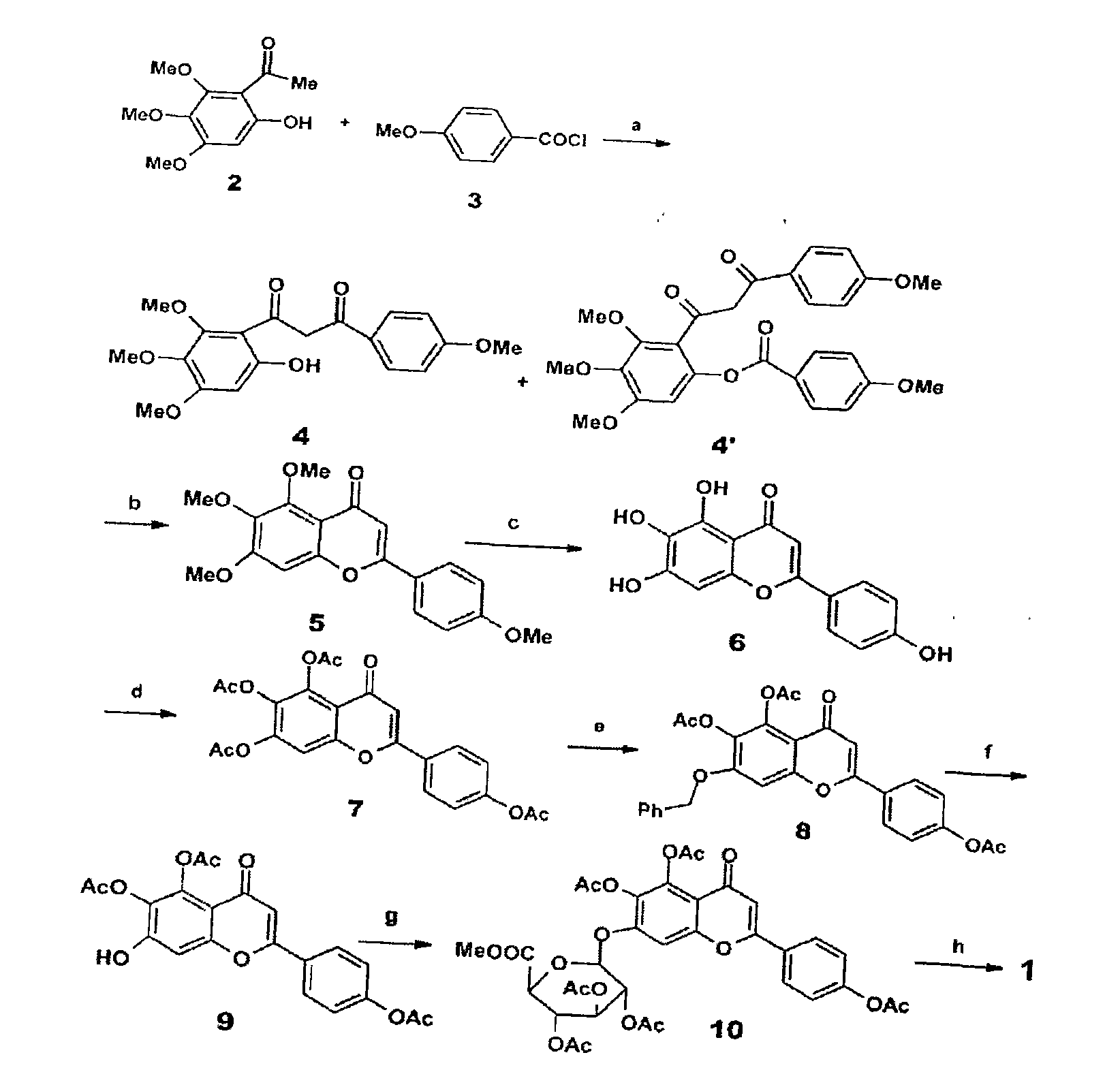
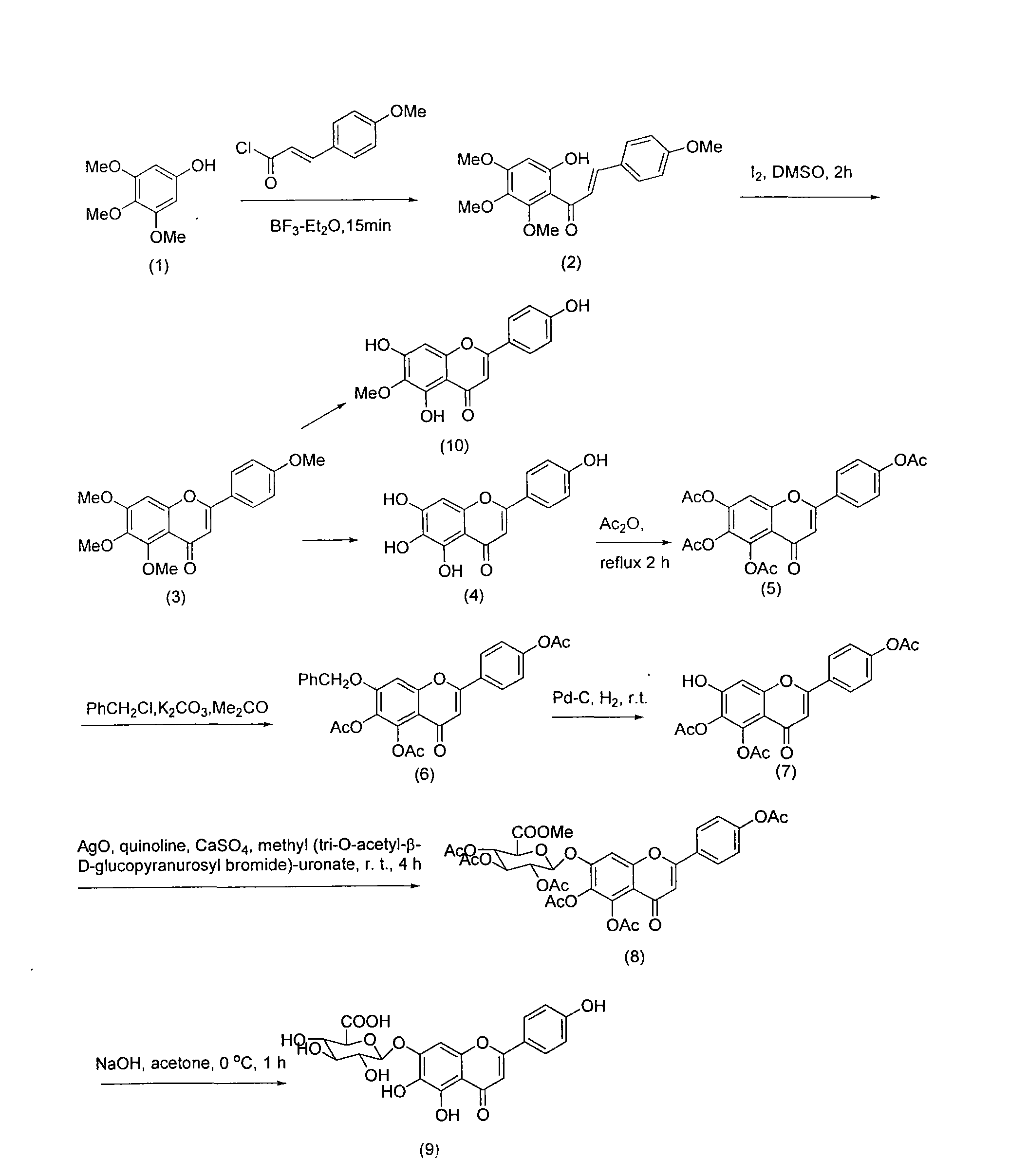
Method for synthesizing 5,6,4'-trihydroxyflavone-7-O-D-glucuronic acid
A technology of trihydroxyflavone and glucuronic acid, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of long synthetic route and low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Take 1.84g 3,4,5-trimethoxyphenol and 2.7g p-methoxycinnamic acid chloride, and place 20mL BF 3 -Et 2 O solution, make it fully dissolved, add 2g 4A molecular sieve, heat to reflux for 15 minutes, stop heating, filter, recrystallize with petroleum ether-ethyl acetate (3:1) to obtain 9-hydroxy-5,6,7,4 '-Tetramethoxychalcone 3.13g, the transfer rate of this step is 91%.
[0017] Take 3.44g 9-hydroxy-5,6,7,4'-tetramethoxychalcone, 200mg iodine, dissolve it in 25mL DMSO, reflux for 2 hours, then carefully pour it into 200g crushed ice, filter, and precipitate Use 20% Na 2 SO 3 It was washed and recrystallized with petroleum ether-ethyl acetate (10:1) to obtain 2.98 g of 5,6,7,4'-tetramethoxyflavonoids with a transfer rate of 87%.
[0018] Take 3.42g of 5,6,7,4'-tetramethoxyflavone, add 5ml of 47% HBr, 10ml of glacial acetic acid, mix well, heat to reflux for 18 hours, then carefully pour 200g of crushed ice to obtain a yellow precipitate. The precipitate was collected by filtra...
Embodiment 2
[0025] Purification of the target product
[0026] Take 100g of the whole product, add 1000mL pure water, adjust the pH value to 7 with 30% sodium hydroxide solution, make it completely dissolved, filter, and add 8 times acetone to the filtrate at 25℃ for precipitation, and stir while adding acetone , Make the precipitation complete, let stand for 12 hours, filter, add acetone to wash three times, then move the precipitate to another container, add 6 times the amount of 40% acetone, stir well, then add 25% hydrochloric acid to adjust the pH to 1 ~2, stand for 10 hours, suction filter, wash with water to neutrality, add ethanol to wash once, and dry to obtain refined products. HPLC analysis showed that the content of 5,6,4'-trihydroxyflavone-7-O-D-glucuronic acid in the obtained sample was 99.25%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com