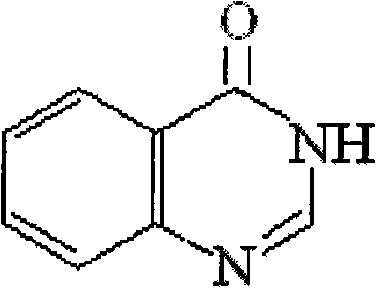
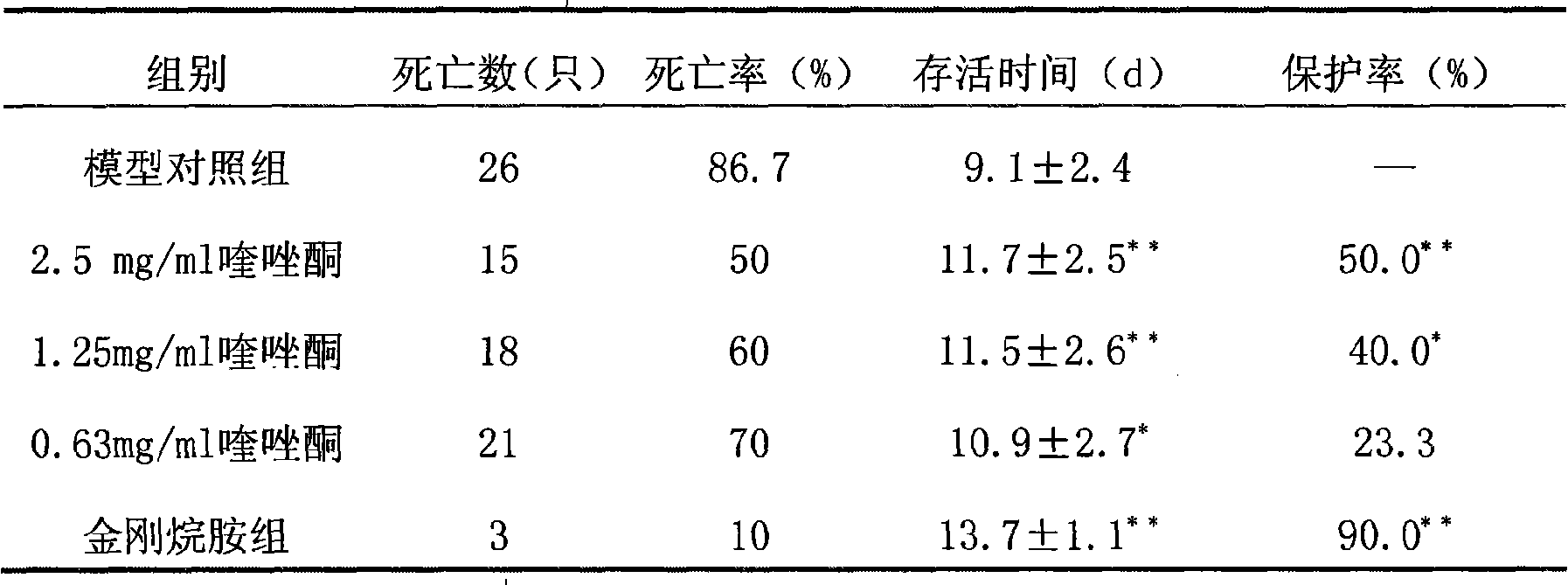
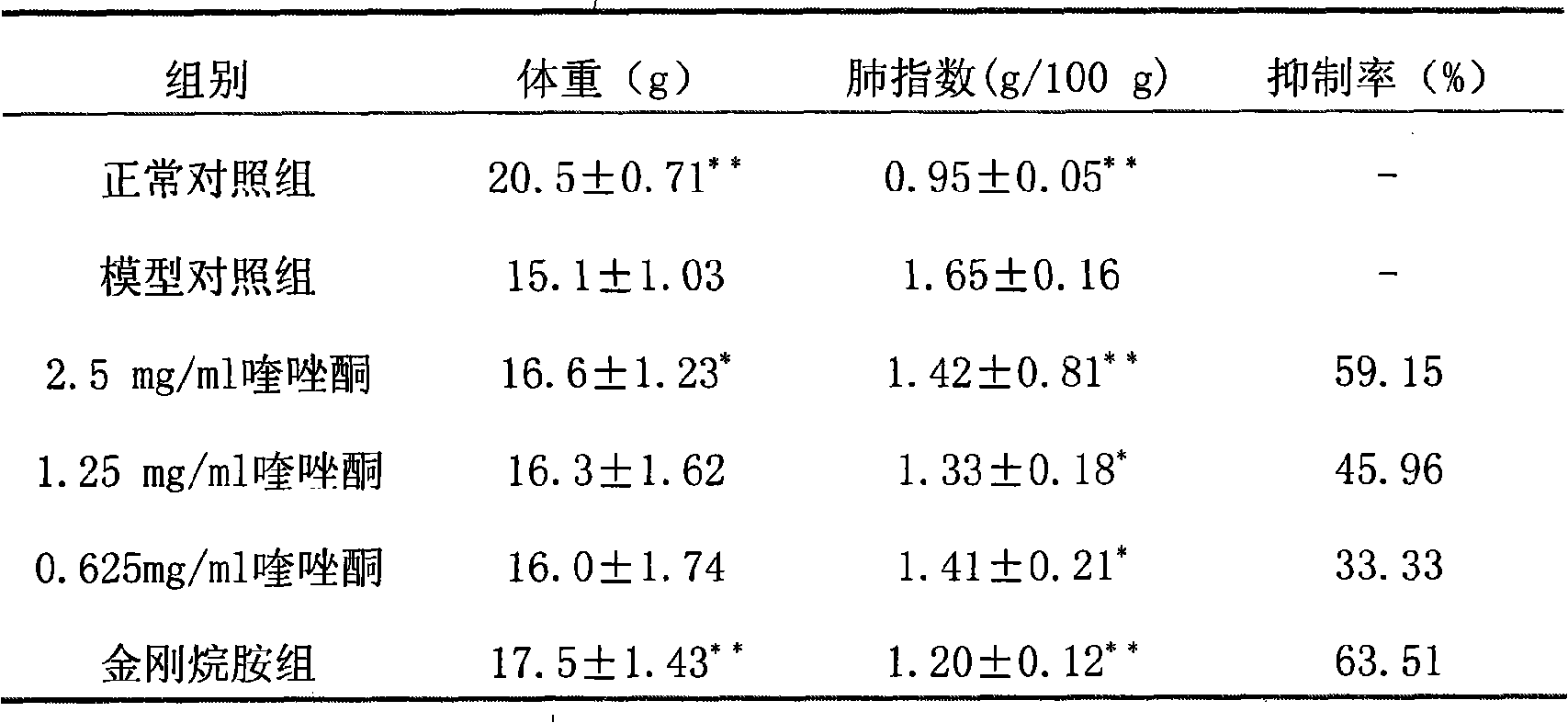
Drug for preventing and curing H1N1 flu virus by adopting 4 (3H) quinazolone
An influenza virus, H1N1 technology, applied in the field of drugs to prevent and treat H1N1 influenza virus, can solve the problems of emergence of drug-resistant strains, large side effects, and poor tolerance of the elderly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0122] The preparation of embodiment 14 (3H) quinazolon tablet
[0123] 4 (3H) quinazolones and the weight ratio of each auxiliary material consumption are as follows:
[0124] 4(3H)quinazolone 1 part
[0125] 1 part microcrystalline cellulose
[0126] Calcium hydrogen phosphate 8 parts
[0127] 10% starch slurry 4 parts
[0128] starch 2 parts
[0129] Carboxymethyl starch sodium 0.4 parts
[0130] Magnesium stearate 0.3 parts
[0131] Preparation method: Folium folium pulverization (40 mesh) - heat reflux with 10 times the amount of 5% ethanol irradiated by microwave (109W) for 10 minutes - concentration - purification (the content of 4(3H) quinazolones is greater than 85%) - drying - crushing and sieving - adding excipients (calcium hydrogen phosphate, starch, microcrystalline cellulose, 10% starch slurry, sodium starch glycolate) - mixing - granulation - drying - granulation - lubricant (magnesium stearate) - mixing - Tablet coating - Packaging
Embodiment 24
[0132] The preparation of embodiment 24 (3H) quinazolon oral liquid
[0133] 4 (3H) quinazolones and the weight ratio of each auxiliary material consumption are as follows:
[0134] Element
[0135] Preparation method: Pulverize Folium Folium (40 mesh) - heat reflux with 10 times the amount of 5% ethanol irradiated by microwave (109W) for 10 minutes - concentration - refining and purification (4(3H) quinazolones content is greater than 85%) - immersion Paste-alcohol precipitation-green paste-water precipitation-adding excipients (purified water, sucrose, honey, sodium benzoate (preservative))-preparation-filtration-filling
Embodiment 34
[0136] The preparation of embodiment 34 (3H) quinazolon granules
[0137] 4 (3H) quinazolones and the weight ratio of each auxiliary material consumption are as follows:
[0138] Element
[0139] Preparation method: Pulverized Folium Folium (40 mesh) - heat reflux with 10 times the amount of 5% ethanol irradiated by microwave (109W) for 10 minutes - concentration - refining (4(3H) quinazolones content is greater than 85%) - adding Excipient sucrose-mixing-soft material-adding excipient dextrin-granulation-drying-adding excipient tartrazine-mixing-packaging
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com