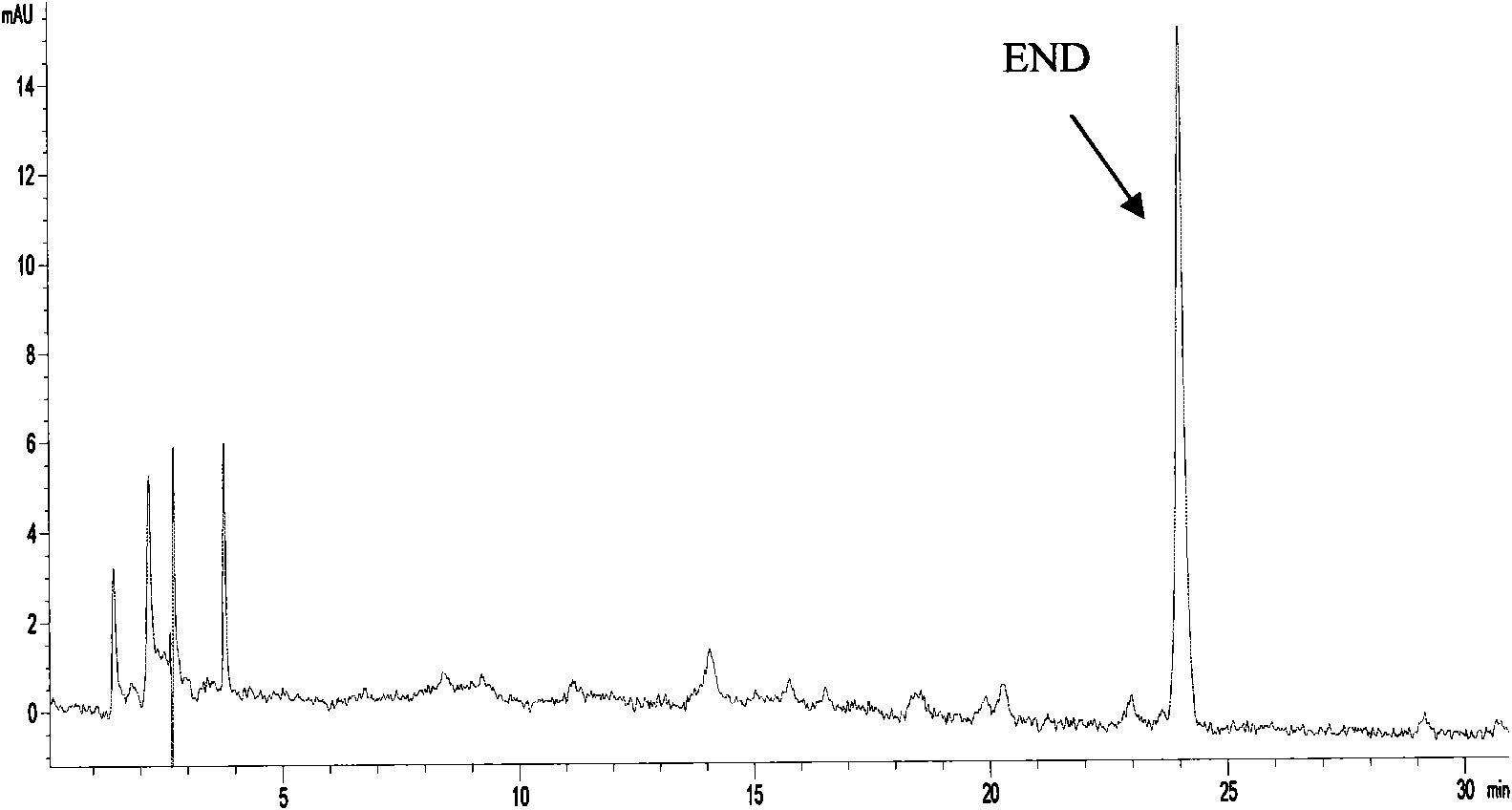
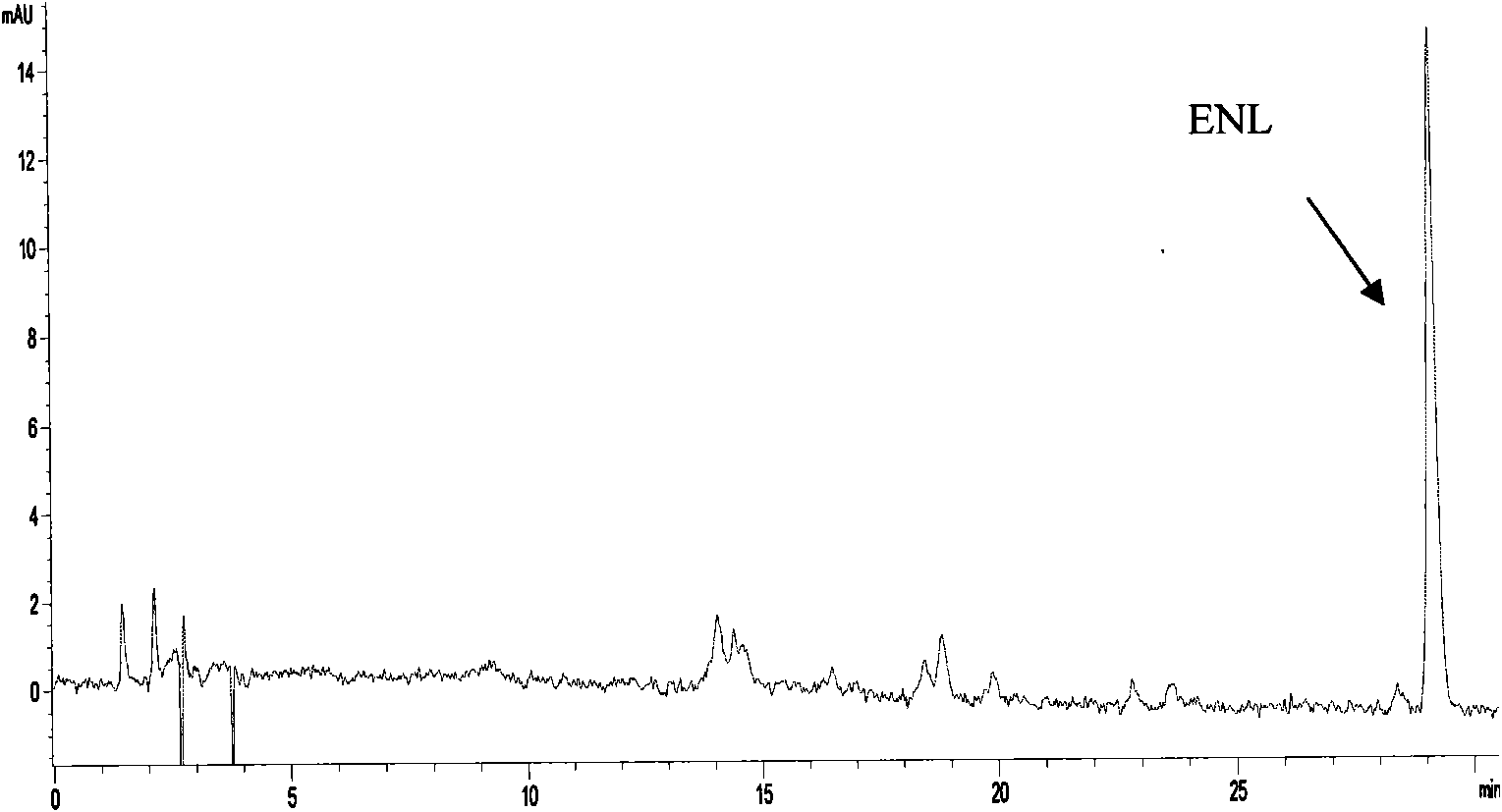
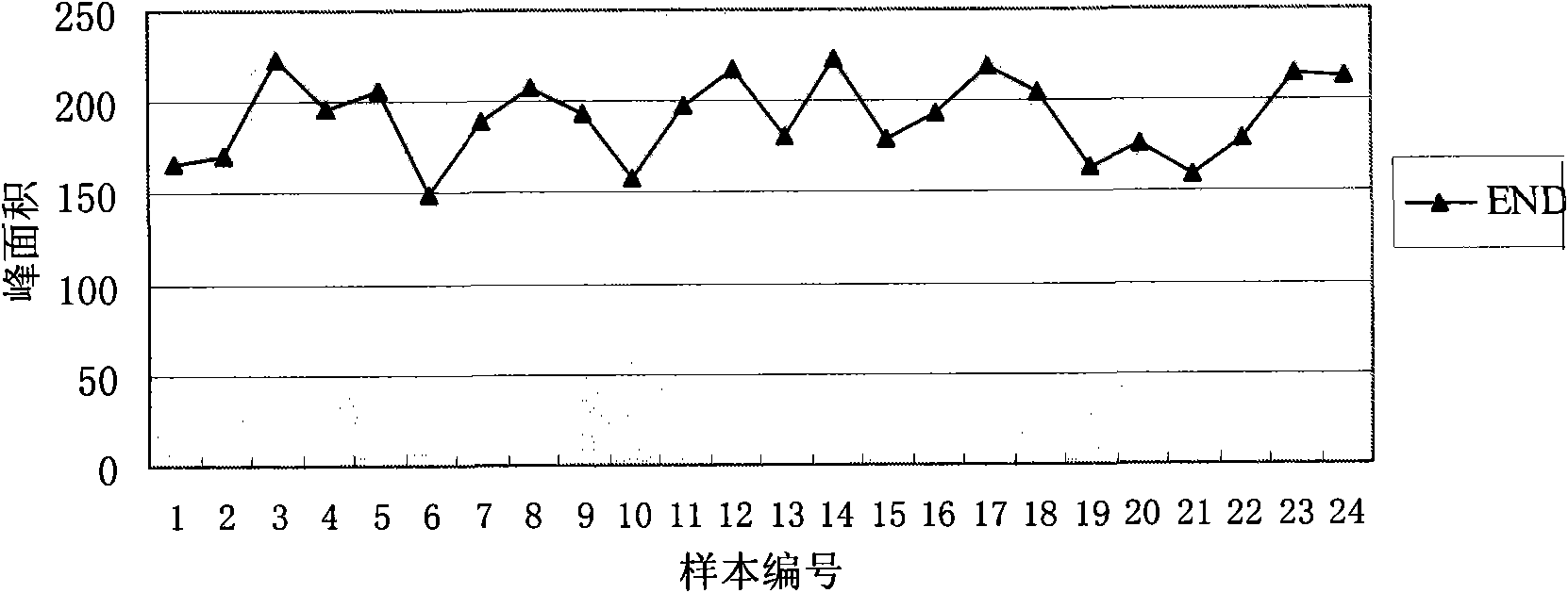
Method of producing enterodiol and enterolactone
A technology of enterodiol and enterolactone, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of inability to produce enterodiol and enterolactone on a large scale, environmental pollution, and high cost of chemical synthesis methods problems, to achieve the effects of low production cost, no environmental pollution, and important scientific value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1. Biotransformation of linseed meal by intestinal bacteria from different human individuals
[0031] 1. Anaerobic medium
[0032] Anaerobic medium (CM605, purchased from Beijing Luqiao Technology Co., Ltd.), its composition is as follows:
[0033] Beef infusion (1000mL); peptone (30g); yeast extract (5g); sodium dihydrogen phosphate (5g); glucose (3g); soluble starch (2g).
[0034] Prepare according to the instructions of the reagent, minced meat slag 5-30g / liter, autoclave at 121°C for 15min.
[0035] 2. Biotransformation
[0036] 1) Take fresh feces from 24 healthy people (see Table 1) and add them to phosphate buffer (pH 7.4) respectively to obtain 24 bacteria solutions with a final concentration of 4g feces / 20mL phosphate buffer.
[0037] 2) Inoculate the 24 kinds of bacterial solutions obtained in step 1 into the anaerobic medium at a ratio of 1:10, and inoculate anaerobically at 37°C (oxygen partial pressure is 3.03975kPa) for 36 hours to enrich the ba...
Embodiment 2
[0045] Example 2: Transformation of linseed meal from human intestinal bacteria liquid
[0046] 1. Prepare culture medium
[0047] 1) Anaerobic medium
[0048] GAM broth medium (Nissui Co., Tokyo, Japan), its composition is as follows:
[0049] 3 grams of soybean peptone; 10 grams of peptone; 13.5 grams of digestive serum powder; 5 grams of yeast extract; 2.2 grams of beef extract; 1.2 grams of bovine liver extract (powder); 3 grams of glucose; KH 2 PO 4 2.5 grams; 5 grams of soluble starch; 0.3 grams of L-cysteine salt; 0.3 grams of sodium thioglycolate; broth (beef heart soup) 1000 ml. Prepare according to reagent instructions. Adjust the pH to 7.2-7.4, autoclave at 10 pounds for 15 minutes.
[0050] 2) Prepare carbon-free medium
[0051] Prepare as follows: add 3g NaCl, 1.0g NH to every liter of phosphate buffer (pH 7.5) 4 Cl, 0.3g L-cysteine hydrochloride; cover with paraffin oil, and autoclave at 121°C for 15min.
[0052] 2. Biotransformation
[0053] 1) Prepa...
Embodiment 3
[0063] Embodiment 3, the impact of the number of passages of human intestinal bacteria on the biotransformation effect
[0064] 1. Prepare culture medium
[0065] 1) Prepare anaerobic medium
[0066] With the step 1 of embodiment 1.
[0067] 2) Prepare carbon-free medium
[0068] Prepare as follows: add 1.5g NaCl, 0.5g NH to every liter of phosphate buffer (pH 7.0) 4 Cl, 0.1g sodium thioglycolate; cover with paraffin oil, and autoclave at 121°C for 15min.
[0069] 2. Strain passage
[0070] 1) Prepare the bacterial liquid of human sample 9 (final concentration is 4g feces / 20ml phosphate buffer), the preparation method is the same as that of step 2 of Example 1, and obtain the bacterial liquid of sample 9.
[0071] 2) Inoculate the bacterial solution of sample 9 into the anaerobic medium at a ratio of 1:10, and perform anaerobic culture at 40° C. (oxygen partial pressure is 7.03975 kPa) for 24 hours to enrich the bacteria.
[0072] 3) Inoculate the enriched bacterial solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com