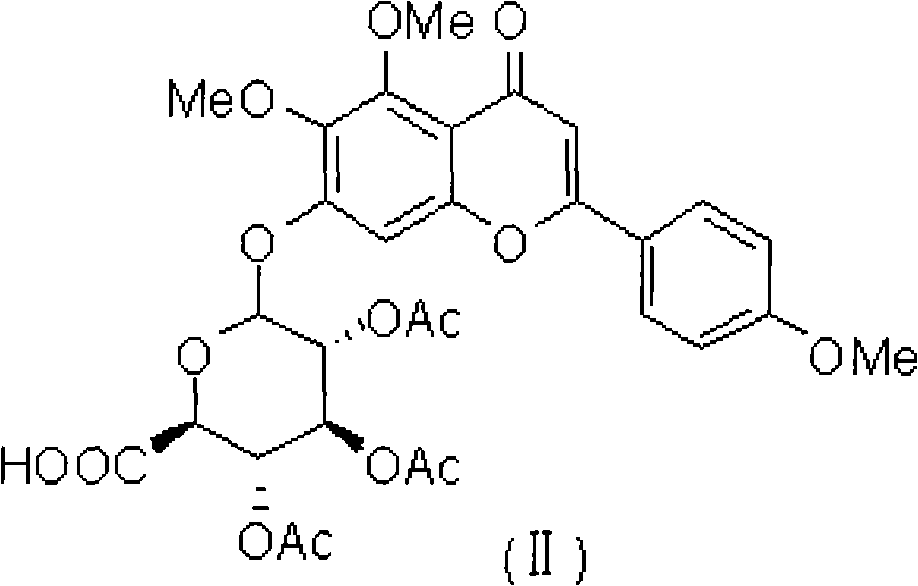
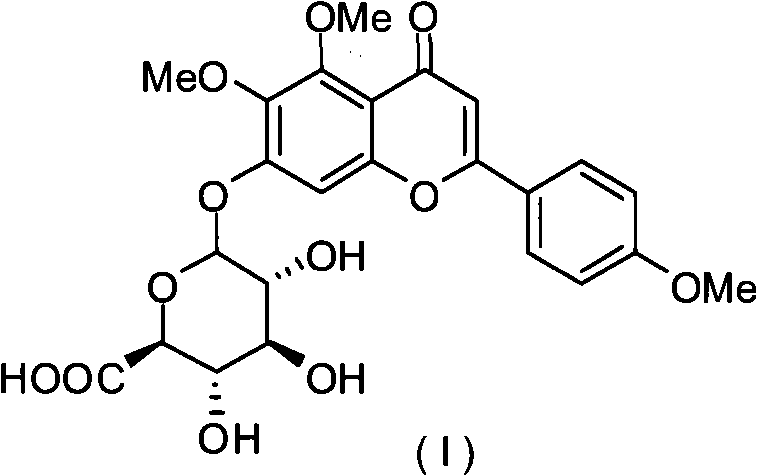
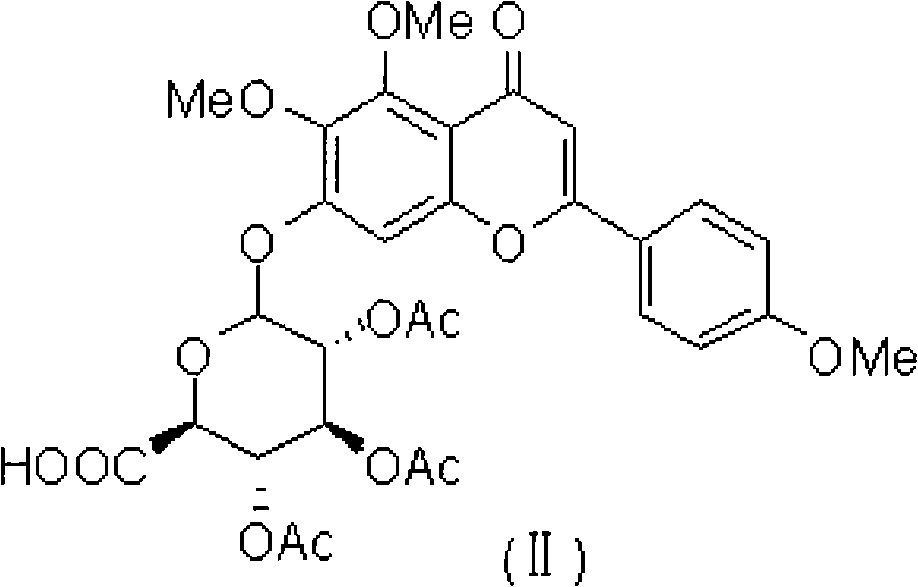
Novel scutellarin derivative as well as preparation method and pharmaceutical composition thereof
A technology of scutellarin and its derivatives, applied in the field of novel scutellarin derivatives, can solve the problem of unsatisfactory therapeutic effect, low bioavailability, dissolution of scutellarin or its derivatives To solve the problems of poor performance and achieve the effects of low cost, easy operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0035] Preparation Example 1 Preparation of A1
[0036] 5 grams (10.8mmol) scutellarin was dissolved in 100 milliliters of DMF and dichloromethane mixed solvent, added 4.6 gram iodomethane and 7.5 grams (54mmol) potassium carbonate, stirred at room temperature for 2 days, TLC (thin layer chromatography ) shows that the reaction raw materials are complete, add 50 milliliters of 1N hydrochloric acid, and extract 3 times with 50 milliliters of ethyl acetate, combine the organic phases, dry with colorless sodium sulfate, spin the organic solvent, and use ethyl acetate / methanol as 1-10: 1 (V / V) was separated and extracted with a silica gel column to obtain 3.1 g of light yellow solid A1 with a yield of 60%. h 1 NMR (400MHz, CDCl 3 )δ7.78(d, 2H), 6.92(d, 2H), 6.60(s, 1H), 6.50(s, 1H), 4.94(d, 1H), 4.01(d, 1H), 3.83(s, 3H ), 3.81(s, 3H), 3.71(s, 3H), 3.47-3.67(m, 5H); C 13 NMR (100MHz, CDCl 3 )δ181.76,181.68,168.14,163.59,163.54,161.79,155.15,152.38,152.10,151.62,132.25,127.28,1...
preparation Embodiment 2
[0037] Preparation Example 2 Preparation of A2
[0038] 504 mg of 4', 5, 6-trimethoxyscutellarin (A1) obtained in Example 1 (dissolved in 10 milliliters of ethyl acetate and dichloromethane mixed solvent, added 0.5 milliliters of acetic anhydride and a drop of pyridine in Stir at room temperature for 6 hours, TLC detects that the reaction of the raw materials is complete, add 20 ml of saturated saline and 10 ml of ethyl acetate, extract and leave the organic phase, dry over anhydrous sodium sulfate, evaporate the solvent to dryness, and use ethyl acetate / petroleum ether by volume The ratio is 1: 1-10, and the silica gel column separation and extraction obtains 421 mg of light yellow product A2, and the yield is 66.8%. H 1 NMR (400MHz, CDCl 3)δ7.86(d, 2H), 7.03(d, 2H), 6.75(s, 1H), 6.59(s, 1H), 5.40(m, 3H), 5.26(d, 1H), 4.25(d, 1H ), 3.90(s, 3H), 3.85(s, 3H), 3.76(s, 3H), 2.11(s, 3H), 2.08(s, 3H), 2.07(s, 3H), C 13 NMR (100MHz, CDCl 3 )δ182.78,169.98,169.36,169.23,166.82,16...
experiment example 1
[0039] Experimental Example 1 Pharmacological Study on the Protective Effect of A2 on Cardiovascular and Cerebrovascular Ischemia
[0040] A large number of pharmacological studies have proved that breviscapine can dilate cerebral blood vessels, reduce cerebral vascular resistance, increase cerebral blood flow, improve microcirculation, improve blood-brain barrier permeability, enhance the phagocytic immune function of macrophages in the body, and resist pituitrin. The result is cerebral ischemia and hypoxia, and can resist platelet aggregation caused by adenosine diphosphate (ADP). Breviscapine is widely used clinically to treat paralysis caused by cerebral thrombosis, cerebral infarction and undetermined types of post-stroke paralysis, hypertension, cerebral embolism, multiple neuritis, chronic arachnoid and other cerebrovascular accidents, as well as coronary heart disease, angina pectoris, Treatment of gouty arthritis and other diseases.
[0041] The activity of compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com