Method for improving performance of direct sodium borohydride fuel cell
A sodium borohydride, fuel cell technology, applied in the field of electrochemical applications, can solve the problems of reducing battery fuel utilization, reducing battery operating voltage, reducing fuel utilization, etc., to reduce charge transfer resistance, improve performance, and inhibit hydrolysis. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Weigh sodium borohydride and dissolve it in 1.5mol / L sodium hydroxide solution to make 0.27mol / L sodium borohydride solution, and then add 0μmol / L; 10μmol / L; 30μmol / L; 50μmol / L of sodium oxalate, mixed well and used as electrolyte for direct sodium borohydride fuel cell. By 0.24cm 2 The platinum sheet is the working electrode, the mercury mercury oxide electrode is the reference electrode, and the graphite rod is the auxiliary electrode. Cyclic voltammetry is used for performance testing.
[0029] Because there are lone pairs of electrons on the carboxyl group in the sodium oxalate molecule, it is easy to adsorb on platinum, the chemical formula is Pt, so that the borohydride on the electrode surface, the chemical symbol is BH 4 - , The combined amount is reduced, and there are enough catalytic points on the electrode to oxidize BH 4 - , So the current of oxidation peak a1 increases. The size of the oxidation peak current changes with the concentration of sodium oxalate....
Embodiment 2
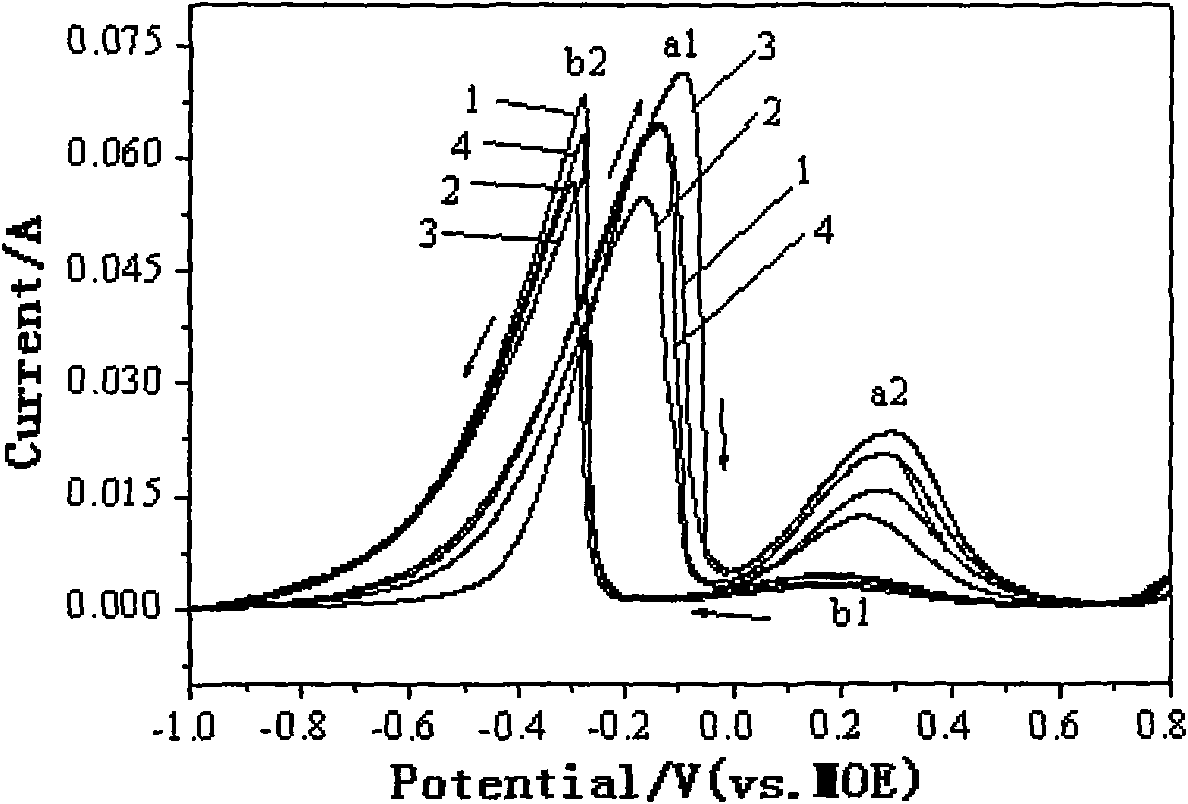
[0031] Weigh sodium borohydride and dissolve it in 1.5mol / L sodium hydroxide solution to make 0.27mol / L sodium borohydride solution, and then add 0μmol / L; 30μmol / L; 60μmol / L; 90μmol / L of urea, mixed well and used as the electrolyte of direct sodium borohydride fuel cell. By 0.24cm 2 The platinum sheet is the working electrode, the mercury mercury oxide electrode is the reference electrode, and the graphite rod is the auxiliary electrode. Cyclic voltammetry and AC impedance spectroscopy at -0.4V activation potential are used for performance testing.
[0032] Cyclic voltammetry test shows that when 60μmol / L of urea is added, the current of oxidation peak a1 directly oxidized by sodium borohydride is the largest, and the current of oxidation peak b2 in the flyback process is the lowest, see figure 2 . In the AC impedance spectroscopy, when adding different concentrations of urea, the electrochemical impedance spectroscopy of sodium borohydride on the platinum electrode is obviously...
Embodiment 3
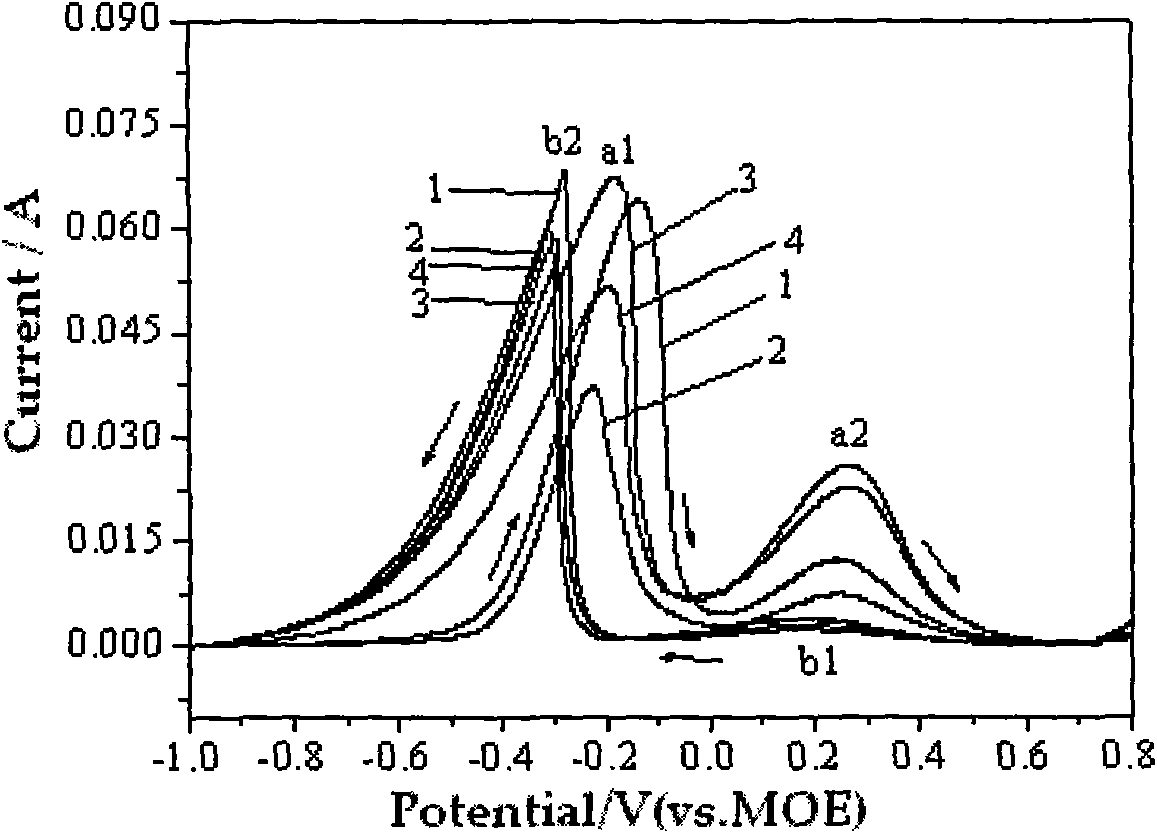
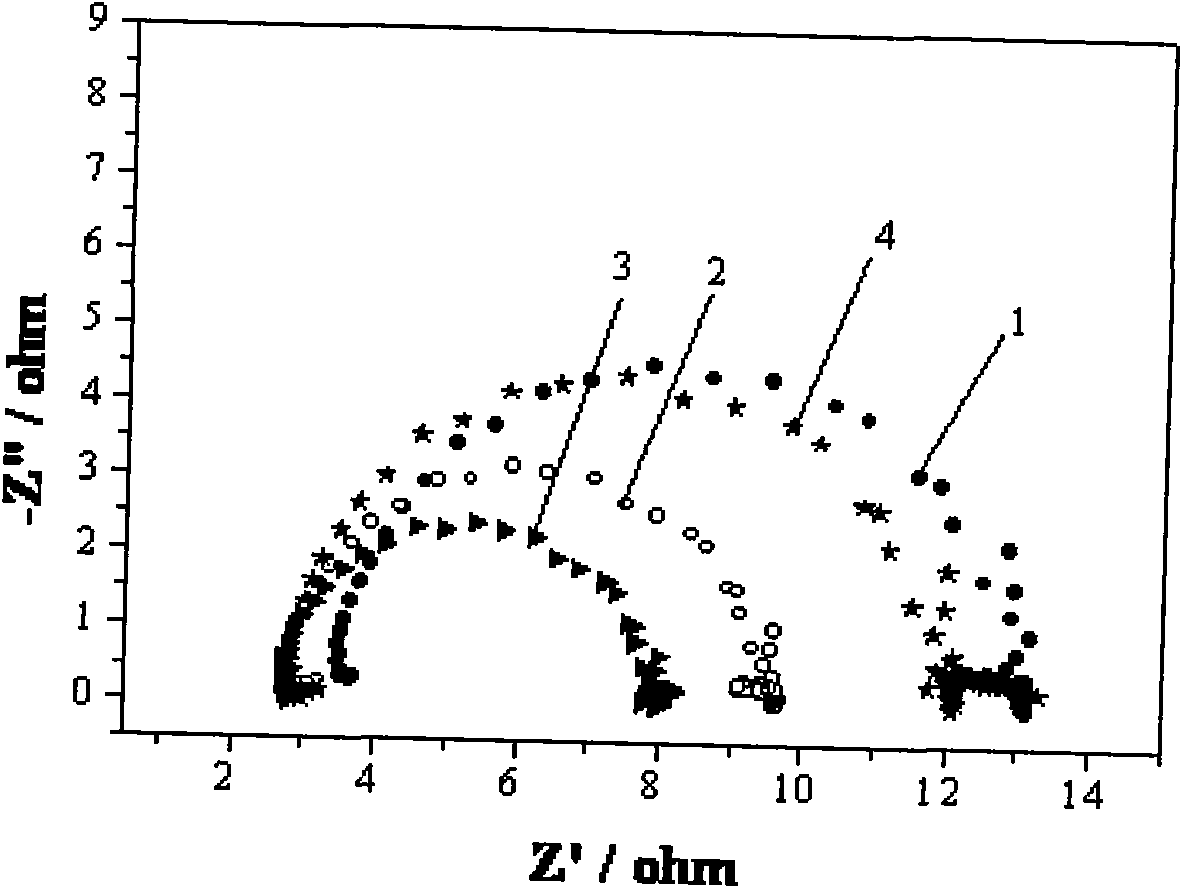
[0035] Weigh sodium borohydride and dissolve it in 1.5mol / L sodium hydroxide solution to make 0.27mol / L sodium borohydride solution, and then add 0μmol / L; 30μmol / L sodium oxalate; 60μmol / L Urea; 0.3μmol / L thiourea; 1mmol / L ethanol, mixed well and used as electrolyte for direct sodium borohydride fuel cell. By 0.24cm 2The platinum sheet is the working electrode, the mercury mercury oxide electrode is the reference electrode, and the graphite rod is the auxiliary electrode. Performance tests are performed by cyclic voltammetry, AC impedance spectroscopy at -0.4V activation potential, open circuit potential, and constant current discharge.
[0036] In AC impedance spectroscopy, the size of the electrochemical impedance arc diameter in the high frequency region is in order: urea Figure 4 . It shows that the addition of 60μmol / L urea can reduce the energy barrier to be overcome in the electrochemical oxidation of sodium borohydride and enhance NaBH 4 -The conductivity of the NaOH solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com