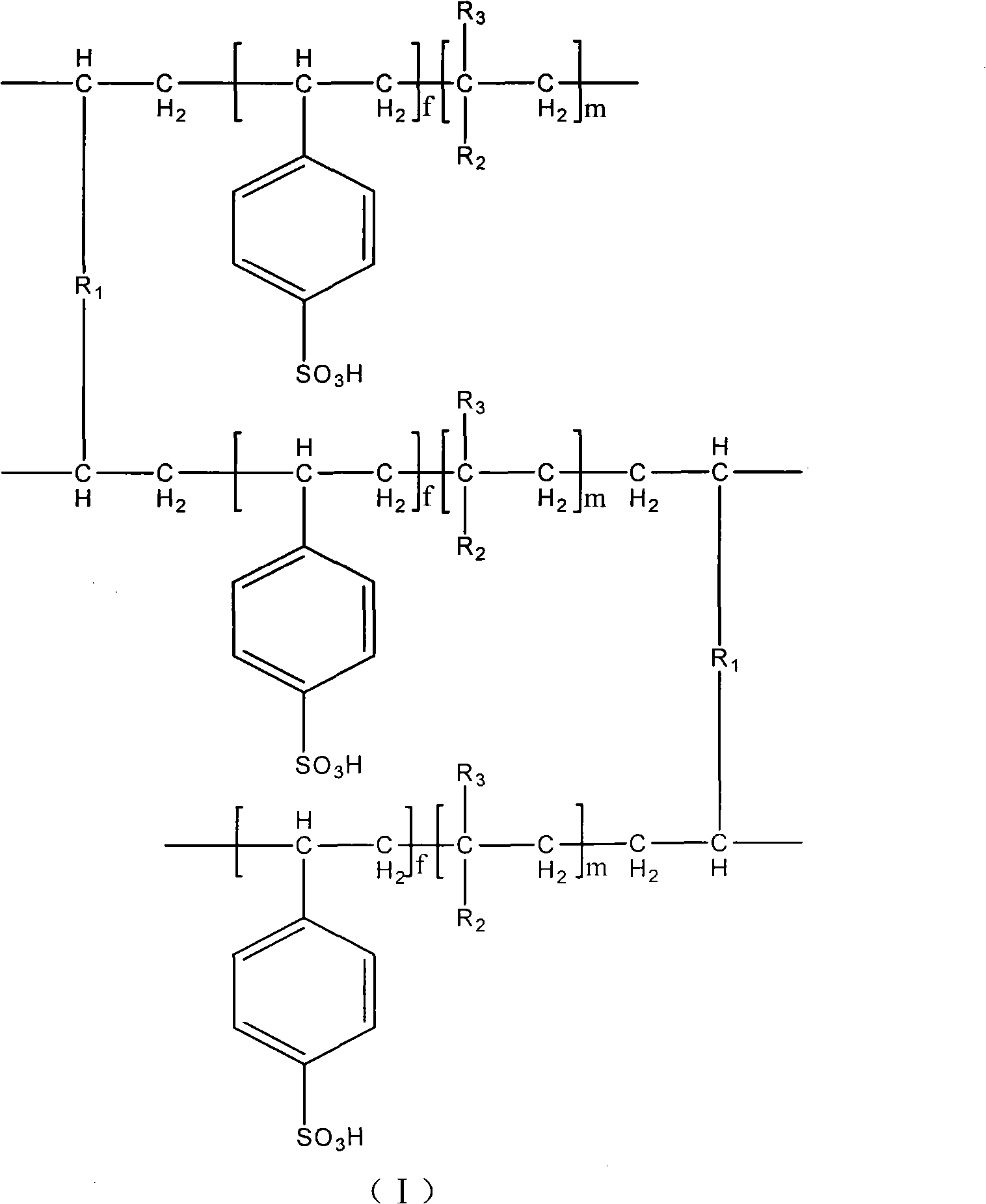
Circular type esterification reaction polymer catalyst and preparation method and application thereof
A technology of esterification reaction and catalyst, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, carboxylate preparation, etc. It can solve the problems of limited number of effective catalysis, limited fixation effect, high toxicity, etc., and achieve good catalysis effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Dispersion polymerization method
[0024] (1) Under the protection of nitrogen, add 2.58 g of sodium styrene sulfonate and 1.08 g of vinyl acetate into a three-necked flask containing 100 mL of dimethyl sulfoxide reaction solvent, and then add 0.0183 g of divinylbenzene Crosslinking agent, 0.0037g initiator benzoyl peroxide and 0.02g polyvinylpyrrolidone as dispersants, and then polymerized at 100℃ with stirring for 2 hours to obtain the dispersion of crosslinked polymer microspheres The system is centrifuged to obtain white solid microspheres of cross-linked polymer.
[0025] (2) Soak the above cross-linked polymer microspheres in saturated saline with twice the volume of the processed cross-linked polymer microspheres for 12 hours, rinse with water for 3 times, and then use 2% sodium hydroxide by mass Soak the solution for 6 hours, rinse the cross-linked polymer microspheres until the drained water is close to neutral, and then soak for 8 hours with a 3% hydroc...
Embodiment 2
[0028] Example 2: Dispersion polymerization method
[0029] (1) Under the protection of nitrogen, add 25.75g of sodium styrene sulfonate and 10.75g of vinyl acetate into a three-necked flask containing 100mL of N-methylpyrrolidone reaction solvent, and then add 5.47g of diethylene glycols Diacrylate (DEGDA) crosslinking agent, 3.65g initiator azobisisobutyronitrile and 2.92g polyvinylpyrrolidone as dispersants, and then polymerize at 80°C for 6 hours with stirring to obtain crosslinking The dispersion system of linked polymer microspheres is centrifuged to obtain white solid microspheres of crosslinked polymer.
[0030] (2) Soak the above cross-linked polymer microspheres in saturated saline with twice the volume of the processed cross-linked polymer microspheres for 20 hours, rinse with water for 5 times, and then use sodium hydroxide with a mass concentration of 10%. Soak the solution for 3 hours, rinse the cross-linked polymer microspheres until the drained water is close to neut...
Embodiment 3
[0032] Example 3: Dispersion polymerization method
[0033] (1) Under the protection of nitrogen, add 5.15g of sodium styrene sulfonate and 2.15g of vinyl acetate into a three-necked flask containing 100mL of N-methylpyrrolidone reaction solvent, and then add 1.09g of polyethylene glycol (600 ) Acrylate (PEG(600)DA) crosslinking agent, 0.73g initiator lauryl peroxide and 5.60g hydroxymethyl cellulose as dispersant, and then polymerize at 110°C with stirring After 4 hours, a dispersion system of cross-linked polymer microspheres was obtained, and then centrifuged to obtain white solid microspheres of cross-linked polymer.
[0034] (2) Soak the above cross-linked polymer microspheres in saturated saline with twice the volume of the processed cross-linked polymer microspheres for 16 hours, rinse with water for 4 times, and then use sodium hydroxide with a mass concentration of 6%. Soak the solution for 5 hours, rinse the cross-linked polymer microspheres until the drained water is cl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com