Phenyloboricacid-modified cationic polymer and composite method and application thereof
A technology of cationic polymers and phenylboronic acid, which is applied in the direction of introducing foreign genetic material using carriers and recombinant DNA technology, etc., can solve the problems of high cost and wide application, lack of safe and effective delivery system for gene drugs, etc., and achieve high-efficiency transfection , high transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
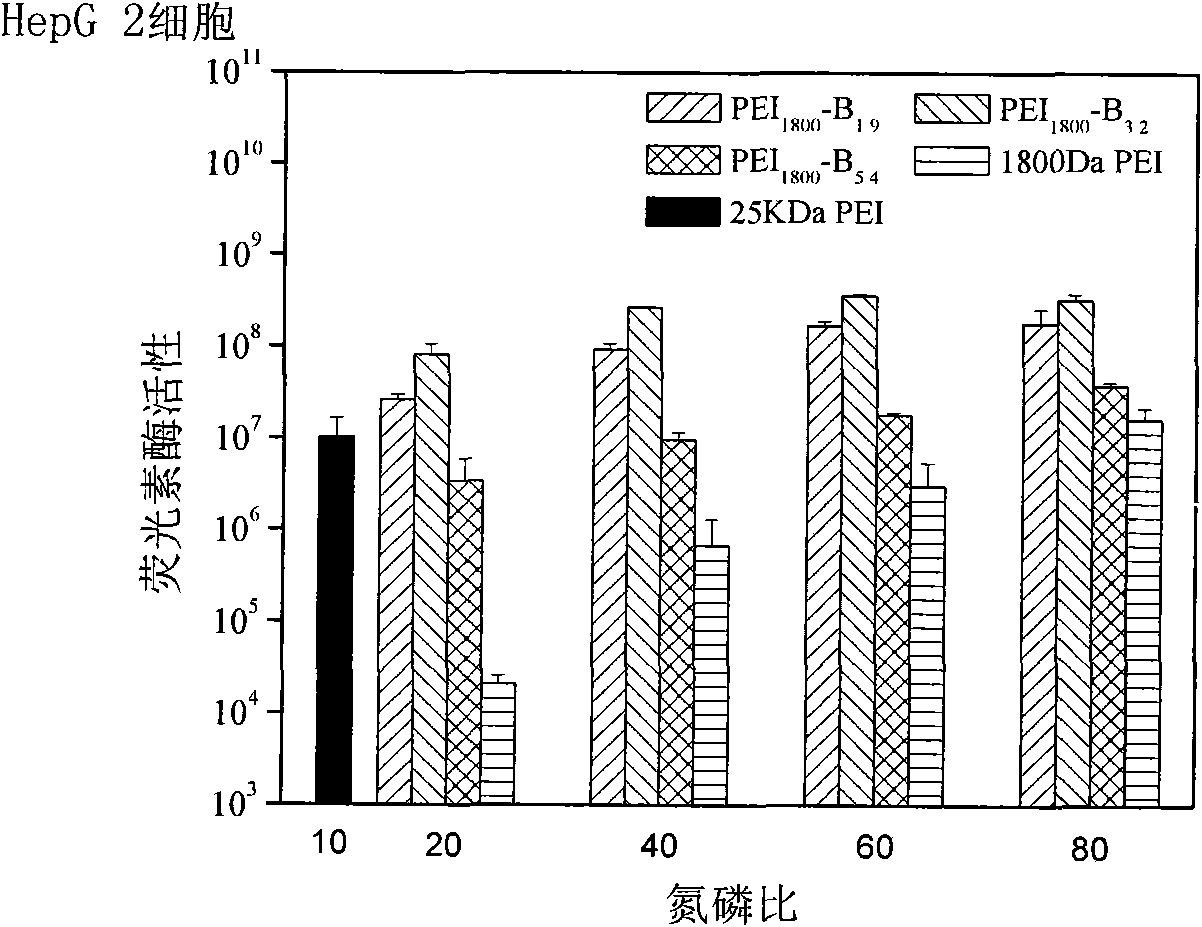
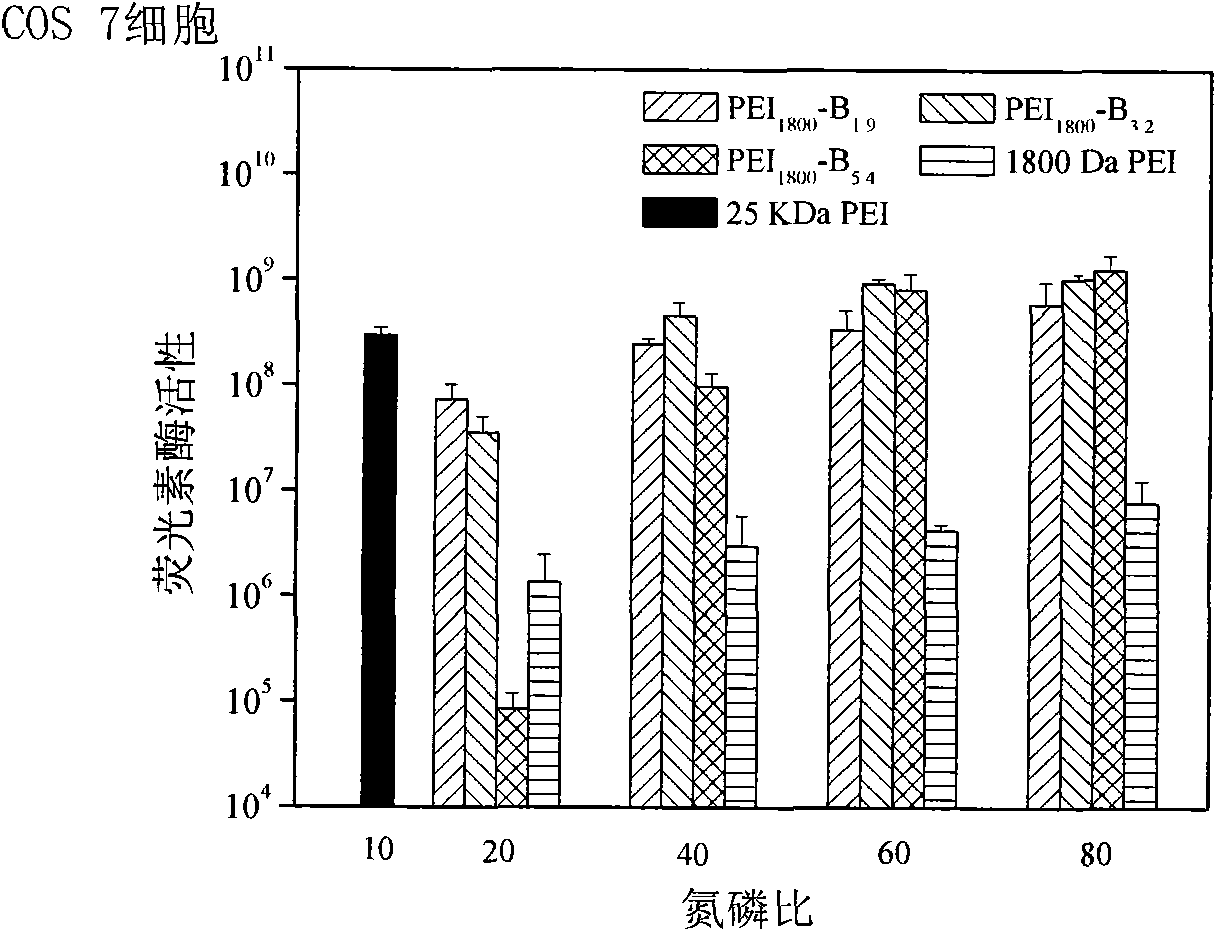
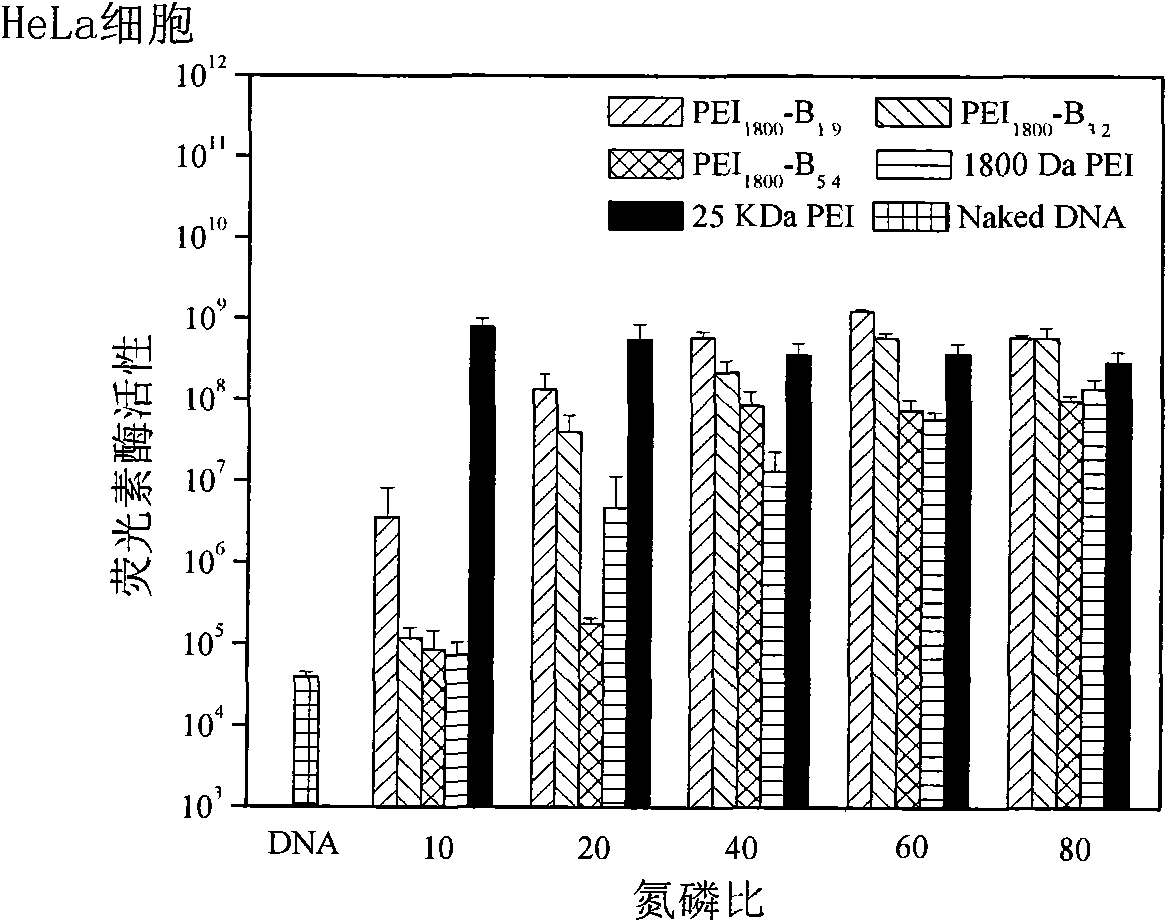
[0026] The present invention will be described in detail below in conjunction with the examples, but not limited to the content disclosed in the examples. Example 1: PEI 1800 -pPB 1.9 (PEI represents that the cationic polymer is polyethyleneimine; numbers such as 1800 represent the molecular weight of polyethyleneimine, the same below; PB represents different types of phenylboronic acid groups on the cationic polymer, and the specific structure of the phenylboronic acid group As described in the examples, the same below; o, m, p represent the relative positions of the R group and the boronic acid group on the phenylboronic acid, which are respectively ortho, meta, and para; 1.9 represents that there are 1.9 benzene on each polyethyleneimine molecule Boric acid group, the same below.)
[0027] Dissolve 1.1mmol of p-(bromomethyl)phenylboronic acid in 20ml of methanol to obtain a methanol solution of phenylboronic acid. Under stirring conditions, add the methanol solution of ph...
Embodiment 2
[0028] Example 2: PEI 1800 -pPB 3.2
[0029] Dissolve 2.2mmol of p-(bromomethyl)phenylboronic acid in 20ml of methanol, add the methanol solution of phenylboronic acid into 15ml of 0.56mmol PEI1800 in methanol under stirring, continue to stir and react at 20°C for 36 hours, and spin down under reduced pressure. steamed to remove most of the methanol, the crude product was added to precipitate in ether, after standing, the upper solution was poured off, the precipitate was collected, and after drying, the polyethyleneimine modified by phenylboronic acid was obtained. 1 H NMR (300MHz, D 2 O): δ7.41(d, 2H), 7.13(d, 2H), 2.68-2.50(m, NCH 2 CH 2 N, NCH 2 CHPB), determined by proton nuclear magnetic resonance spectrum, the final product is PEI 1800 -pPB 3.2 .
Embodiment 3
[0030] Example 3: PEI 1800 -pPB 5.4
[0031] Dissolve 4.4mmol of p-(bromomethyl)phenylboronic acid in 40ml of methanol, add the methanol solution of phenylboronic acid to 15ml of 0.56mmol PEI1800 in methanol under stirring, continue to stir and react at 50°C for 18 hours, and rotate under reduced pressure steamed to remove most of the methanol, the crude product was added to precipitate in ether, after standing, the upper solution was poured off, the precipitate was collected, and after drying, the polyethyleneimine modified by phenylboronic acid was obtained. 1 H NMR (300MHz, D 2 O): δ7.38(d, 2H), 7.10(d, 2H), 2.65-2.45(m, NCH 2 CH 2 N, NCH 2 CHPB), determined by proton nuclear magnetic resonance spectrum, the final product is PEI 1800 -pPB 5.4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com