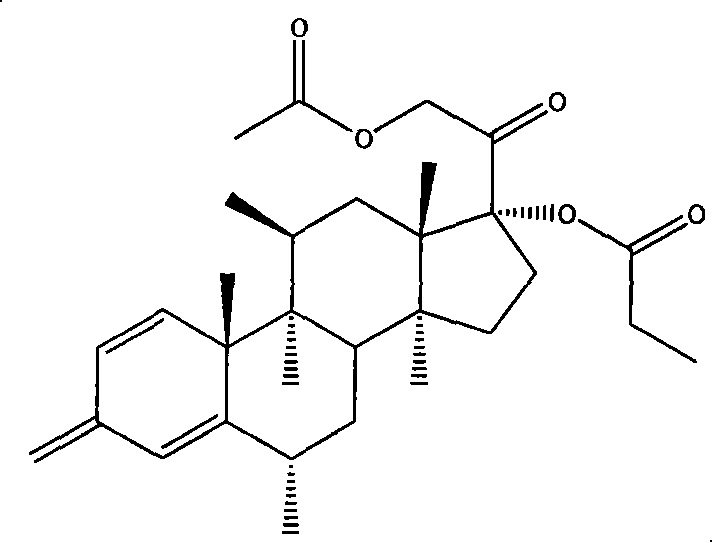
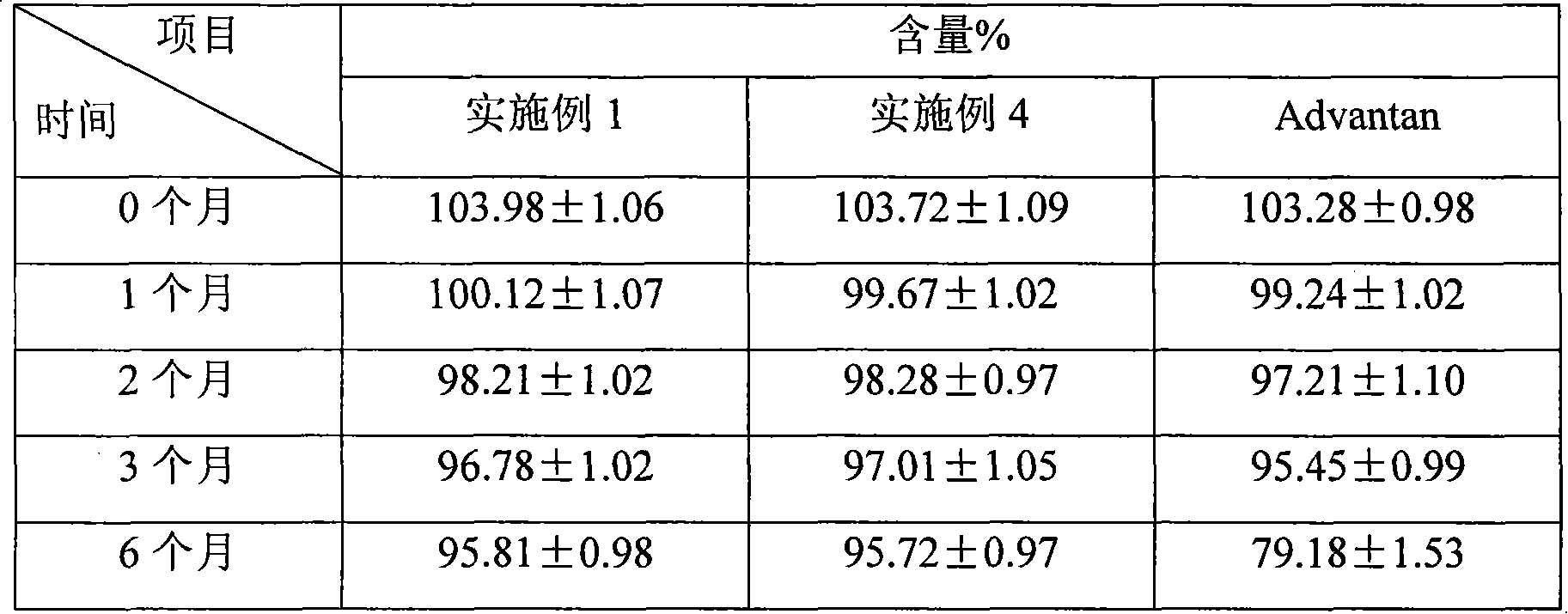
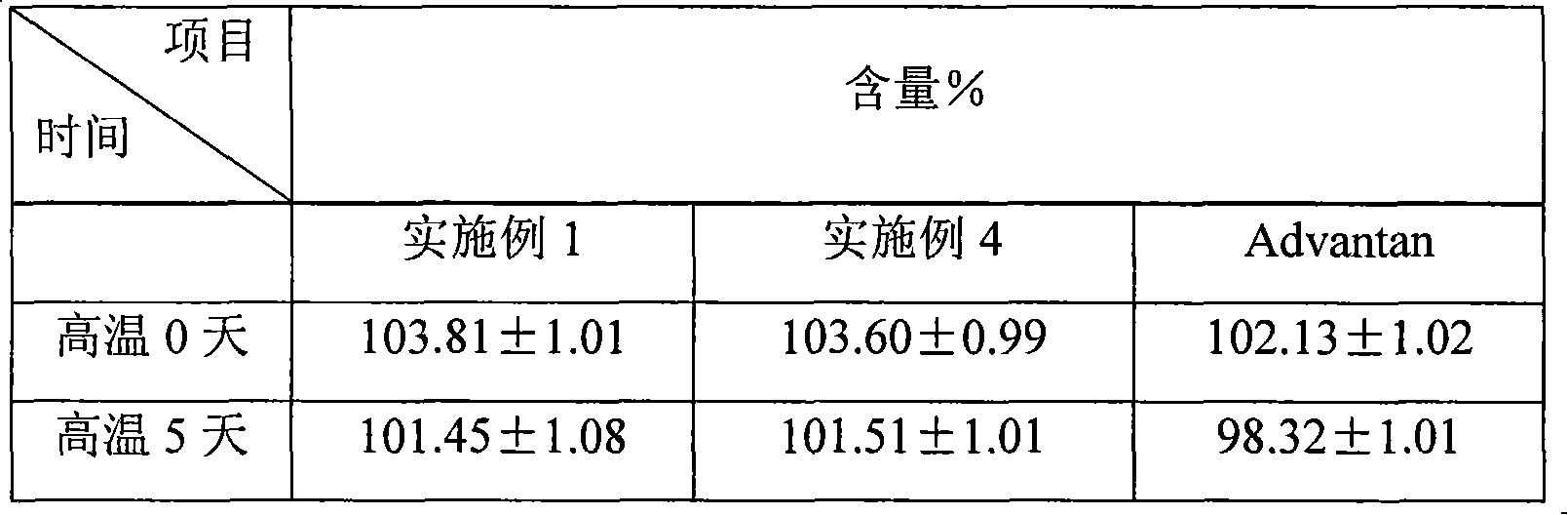
Methylprednisolone aceponate lipidosome cream
A technology of methylprednisolone acetate and liposome, which is applied in the direction of liposome delivery, oil/fat/wax non-active ingredients, medical preparations of non-active ingredients, etc., which can solve the problem of affecting drug penetration and absorption, and cannot fully exert Advantages of liposome administration for external use on the skin, difficulties in encapsulating glucocorticoids into liposomes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Recipe for the oil phase:
[0041] White Vaseline 100g Stearyl Alcohol 30g Liquid Paraffin 50g Glyceryl Monostearate 20g Pingpingjia a-2020g Methylparaben 0.5g Propylparaben 0.05g
[0042] The formula of liposome solution:
[0043] Methylprednisolone acetate 1g, soybean lecithin 20g, cholesterol 2g, disodium edetate (EDTA-2Na) 1g, α-tocopherol 1g
[0044] Glycerin 50g
[0045] The liposome solution is buffered to a phosphate buffer of pH=6.5 Distilled water to 1000g (by total weight of the composition)
[0046] Preparation:
[0047] (1) Preparation of oil phase
[0048] Take the prescribed amount of oil phase ingredients and heat to 70°C, add paraben dissolved in an appropriate amount of organic solvent, then take the prescribed amount of moisturizer and heat it to the same temperature and slowly add it to the oil phase, and stir until it is uniform while adding. have to.
[0049] (2) Preparation of liposome solution
[0050] Weigh the prescribed amount of methyl...
Embodiment 2
[0054] Oil Phase Formula:
[0055] White Vaseline 120g Stearyl Alcohol 50g Liquid Paraffin 50g Glyceryl Monostearate 40g Pingpingjia a-2020g Methylparaben 0.5g Propylparaben 0.05g
[0056] Formulation of liposome solution
[0057] Methylprednisolone acetate 0.5g Hydrogenated soybean lecithin 10g Cholesterol g Disodium edetate 1g
[0058] The liposome solution is buffered to a phosphate buffer of pH=6.5 Distilled water to 1000g (by total weight of the composition)
[0059] The compound method of embodiment 1 makes 0.05% methylprednisolone acetone liposome emulsifiable cream.
Embodiment 3
[0061] Oil Phase Formula:
[0062] White Vaseline 60g Cetyl Alcohol 40g Octadecanol 40g Liquid Paraffin 90g Glyceryl Monostearate 20g Pingpinga-2040g Methylparaben 0.5g Propylparaben 0.05g
[0063] Liposome Solution Formulation
[0064] Methylprednisolone Acetate 0.25g Egg Yolk Lecithin 3g Cholesterol 0.6g
[0065] The liposome solution is buffered to a phosphate buffer of pH=6.5 Distilled water to 1000g (by total weight of the composition)
[0066] The compound method of embodiment 1 makes 0.025% methylprednisolone acetone liposome emulsifiable cream.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com