Polyethylene glycol-modified calcitonin
A kind of polyethylene glycol and calcitonin technology, applied in the field of polyethylene glycol modified calcitonin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
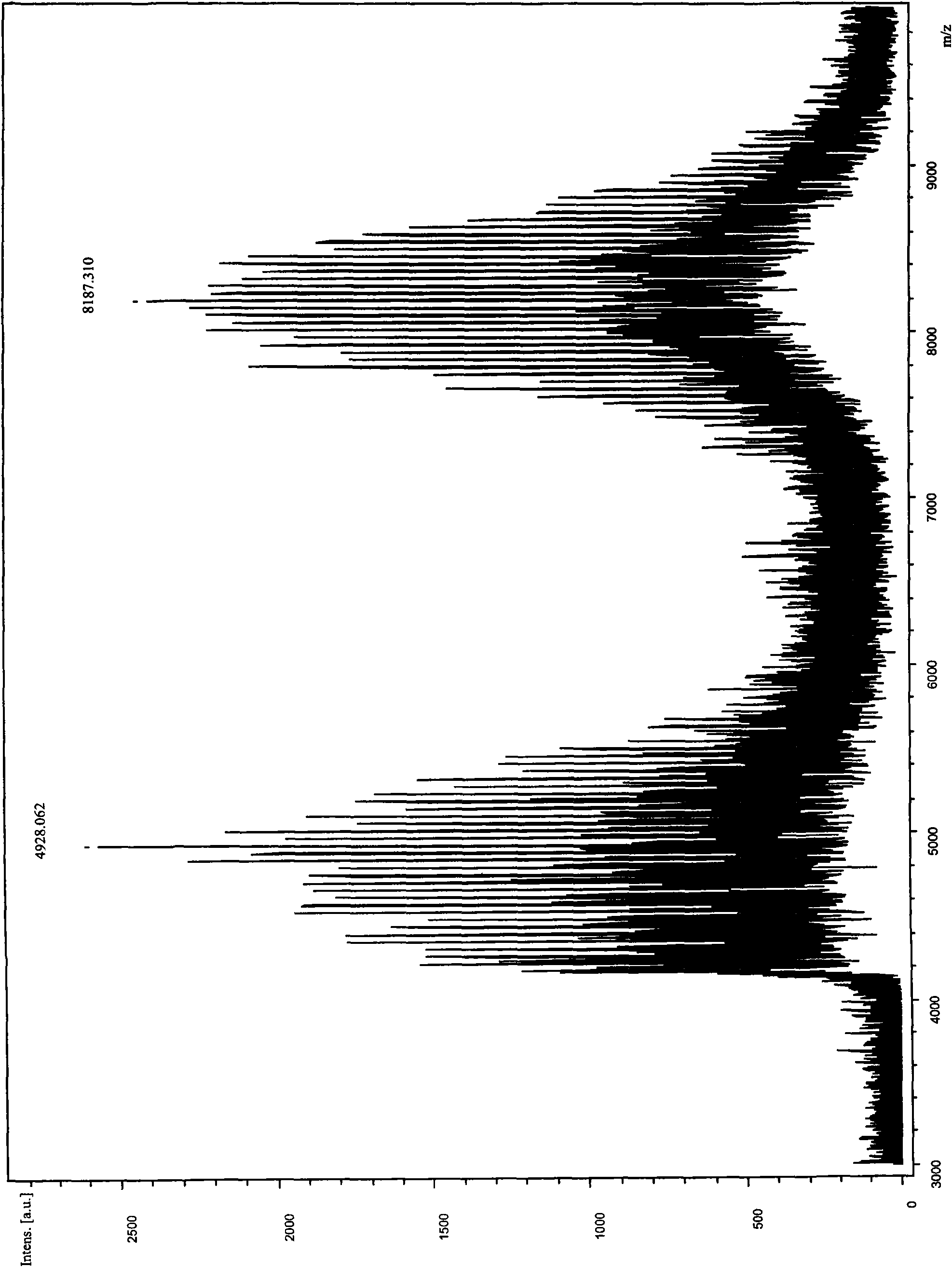
[0044] Synthesis of Salmon Calcitonin Modified by Methoxypolyethylene Glycol Succinimidyl Propionate 5000 (mPEG-SPA-5000)
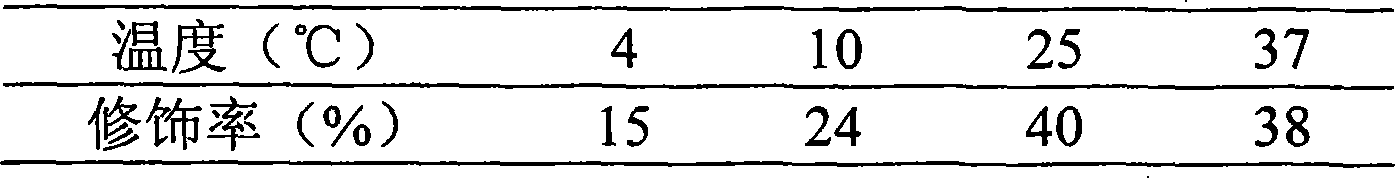
[0045] Choice of reaction temperature: Take 2ml of 1.0mg / ml salmon calcitonin solution, add 2ml of phosphate buffer to make the pH of the solution 7.0, then add 15mg of mPEG-SPA-5000 solid, dissolve, mix well, each take 0.8 ml was placed in 4 test tubes with stoppers, and then placed at 4°C, 10°C, 25°C and 37°C for 30 minutes respectively, and then 5 mg of glycine was added to terminate the reaction. The modification rate of salmon calcitonin modified with single polyethylene glycol (one molecule of polyethylene glycol chain connected to one salmon calcitonin molecule, hereinafter referred to as polyethylene glycol modified salmon calcitonin) was compared to determine the modification conditions. The results show that the PEG-modified salmon calcitonin can be obtained at these temperatures, and the modification rate is the highest at 25°C. See Table 1 for...
Embodiment 2
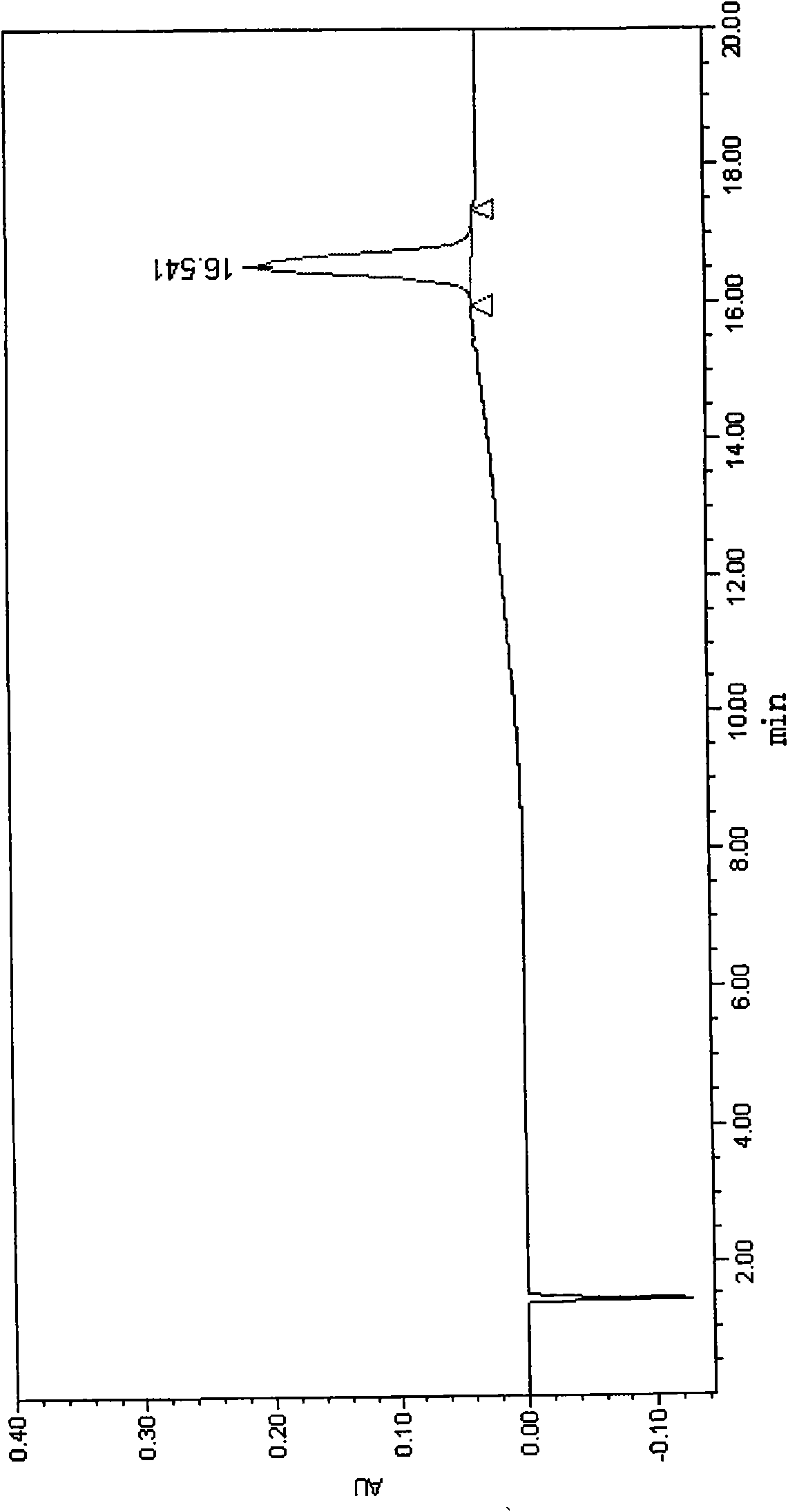
[0055] Purification and Identification of Polyethylene Glycol Modified Salmon Calcitonin
[0056] Take 10ml of 1.0mg / ml salmon calcitonin solution, add 10ml of phosphate buffer to make the pH of the solution 7.0, then add 44mg of mPEG-SPA-5000 solid, dissolve, mix well, react at 25°C for 30min, add 25 mg glycine terminated the reaction.
[0057] Take the above-mentioned reaction solution, use an ultrafiltration membrane with a cut-off molecular weight of 1000, replace it with 0.01mol / L, pH4.0 acetic acid-sodium acetate buffer solution, concentrate to 5ml, and put it on the column for separation. The chromatographic conditions are as follows:
[0058] Chromatography medium: SOURCE 30S
[0059] Column volume: 5ml
[0060] Flow rate: 3.0ml / min
[0061] Column equilibration: Equilibrate 5 times column volume with 0.01mol / L, pH4.0 acetic acid-sodium acetate (initial buffer)
[0062] Sample volume: 5ml
[0063] Elution: first use 3 times the column volume of the initial buffer t...
Embodiment 3
[0077] Salmon calcitonin and polyethylene glycol modified salmon calcitonin (prepared by the method of Example 2) reduce the experimental comparison of rat blood calcium
[0078] With reference to the potency determination method of calcitonin in the two appendix XII O of Chinese Pharmacopoeia in 2005, 90 Wistar female rats with a body weight of 200 ± 15g were healthy and qualified, fasted for 16 hours before the test, drank distilled water freely, and were randomly divided into 3 groups, 30 rats in each of blank, salmon calcitonin and polyethylene glycol modified salmon calcitonin groups. Abdominal subcutaneous injection, blank group injection of normal saline, salmon calcitonin and polyethylene glycol modified salmon calcitonin doses were 0.05 μg / kg (salmon calcitonin in polyethylene glycol modified salmon calcitonin Calculated according to the amount of calcitonin), the administration volume is 0.4ml / 100g. Before the administration and 1, 2, 4, 8, and 12 hours after the ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com