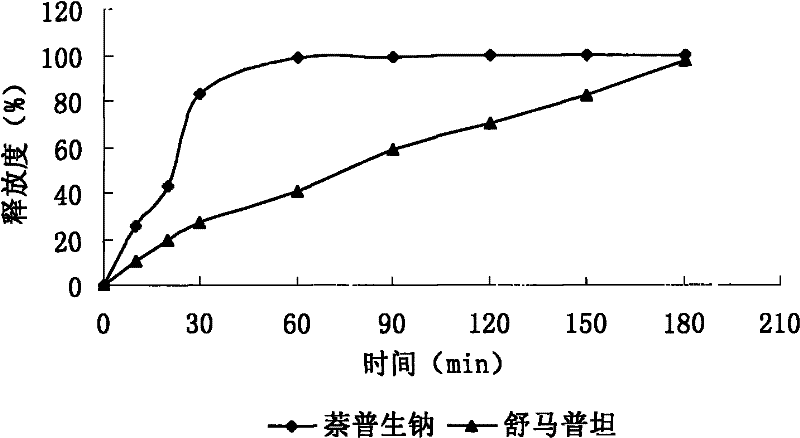
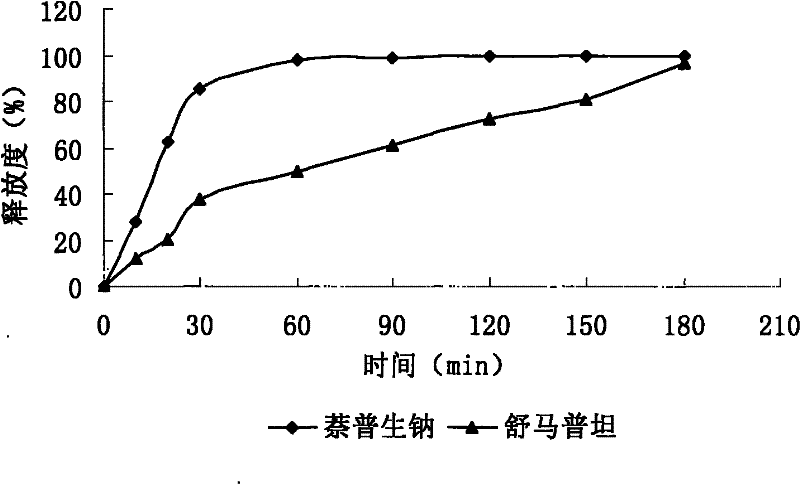
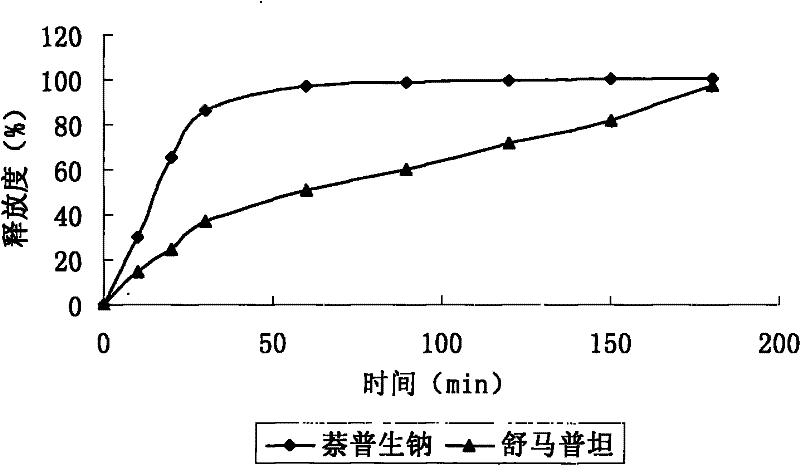
Sumatriptan succinate compound preparation and preparation method thereof
A technology for sumatriptan succinate and compound preparations, which is applied in the field of sumatriptan succinate compound preparations and its preparation, and can solve the problem of affecting the gastrointestinal absorption of non-steroidal anti-inflammatory drugs naproxen sodium, double-layer tablets High equipment requirements, affecting drug release and other issues, to achieve excellent therapeutic effects, low equipment requirements, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1), preparation of sumatriptan succinate pellets:
[0040] Sumatriptan Succinate 119g
[0041] Blank ball core (sucrose type p6, purchased from Hangzhou Gaocheng Bio-Nutrition Technology Co., Ltd.) 100g
[0042] Isolation layer raw material 5% Opadry water dispersion in mass percent
[0043] Coating layer raw material 4% Surelease water dispersion in mass percentage concentration
[0044] Preparation Process:
[0045] Screen blank ball cores between 30-35 meshes, place them in a fluidized bed, prepare an aqueous solution of sumatriptan succinate with a mass percent concentration of 30%, apply medicine, and make the pellets increase in weight by 50%. Bardai water dispersion is used as the raw material of the isolation layer to coat the core of the pill, so that the weight of the pill is increased by 1%, and then Surelease water dispersion is used as the raw material of the coating layer, so that the weight of the pill is increased by 10%, and it is aged at 40°C for 1...
Embodiment 2
[0060] (1) Preparation of sumatriptan succinate pellets:
[0061] Sumatriptan Succinate 119g
[0062] Pill core excipients microcrystalline cellulose 120g
[0063] The raw material of the isolation layer has a mass percentage concentration of 2% hypromellose aqueous solution
[0064] Raw material for coating layer 10% Opadry yellow water dispersion in mass percent
[0065] Preparation process: the amount of sumatriptan succinate and microcrystalline cellulose are respectively pulverized through a 100-mesh sieve, mixed uniformly, and 2% hypromellose is used as a binder to obtain a soft material. Put the prepared soft material in the extrusion spheronizer, the extrusion speed is 40r / min, the spheronization speed is 900rpm, the spheronization time is 5min, dry at 50°C, and take the pellets between 20-40 mesh as the pills core. The prepared drug-containing pellet cores are placed in a fluidized bed, and 2% hypromellose aqueous solution is used as the raw material of the isolat...
Embodiment 3
[0079] (1) Preparation of sumatriptan succinate pellets:
[0080] Sumatriptan Succinate 119g
[0081] Pill core accessories Starch 42.5g Sucrose 42.5g
[0082] The raw material of the isolation layer is 5% Eudragit E100 and 2% stearic acid dissolved in mass percent
[0083] Ethanol / water (1 / 1, v / v) solution of magnesium and 6% polyethylene glycol
[0084] Coating layer raw material mass percent concentration is 2% Surelease water dispersion
[0085] Preparation process: use the above-mentioned amount of starch, sucrose, and sumatriptan succinate to make a wet material with 70% ethanol solution, extrude the wet material to granulate, put it in a spheronizer and spheronize it, and then coat the raw material of the isolation layer. Make the pellets containing 10% increase in weight, and then coat the raw materials of the coating layer to increase the weight by 1%, aging at 50° C. for 24 hours to obtain the sumatriptan succinate pellets. The weight composition of the obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com