Recombinant plancenta hominis globin renaturation and medicine application protecting alcoholic hepatic injury thereof
A technology of cytoglobin and alcohol, which is applied in the prevention or treatment of alcoholic acute liver injury and the preparation of recombinant human cytoglobin expression renaturation, which can solve problems such as complex spatial structure and achieve high resolution, high chemical stability, The effect of good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
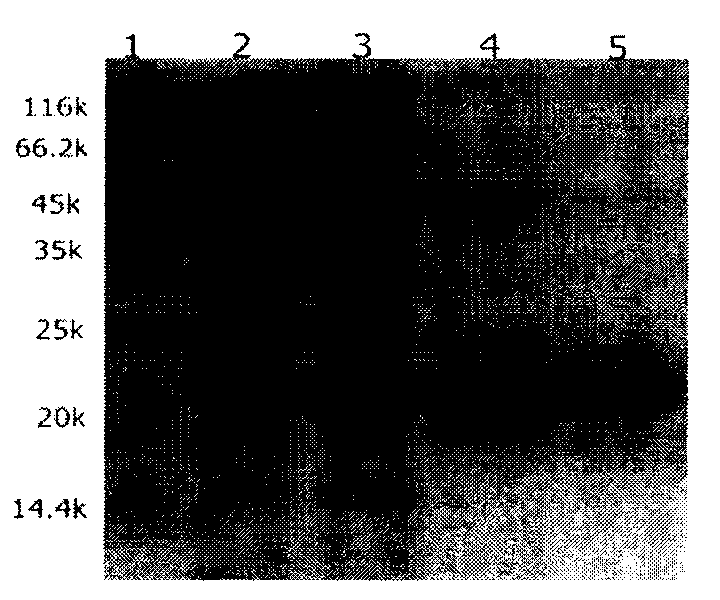
Image
Examples
Embodiment 1
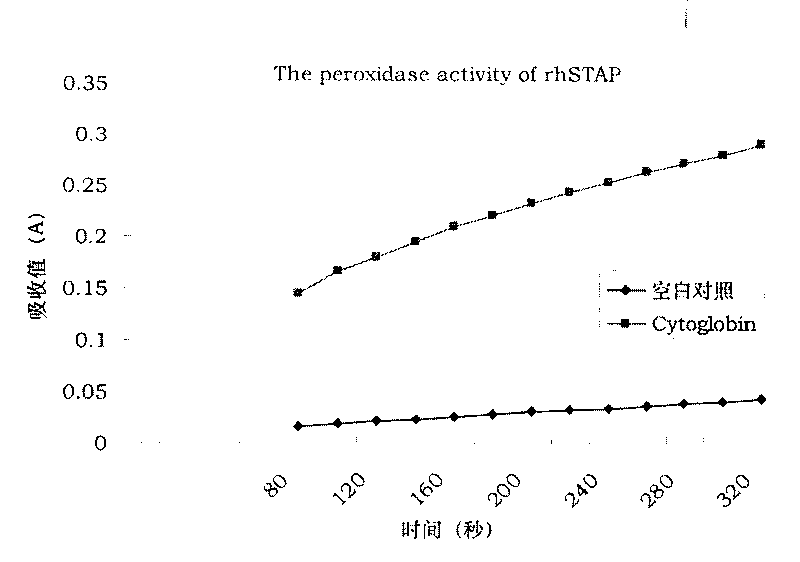
[0024] The activity measurement of the obtained rhCYGB peroxidase of the present invention, get 2 parts of 3ml 5% pyrogallic acid (pyrogall) solution and 0.5% H respectively 2 o 2 100 μl was mixed, and after the A420 absorption value was determined to be no longer increased on a visible spectrophotometer, 100 μl (1 mg / ml) of rhCYGB solution was added, and the other portion was added with the same volume of buffer as a control. The A420 absorbance value of the enzyme-catalyzed product purpurogallin was measured every 20 seconds, and the measurement was continued for 5 minutes. by literature [10] The method was used to analyze and calculate the enzyme activity unit. Under the standard measurement conditions, the amount of enzyme needed to produce 1 mg of rubigallol within 20 seconds was 1 enzyme activity unit. Peroxidase activity analysis showed that the purified rhCYGB protease activity unit was 5u / mg, see image 3 .
Embodiment 2
[0026] Use an ultraviolet scanner (Shimadzu spectrophotometer UV-1700) to record the ultraviolet absorption spectrum of rhCYGB (0.5mg / ml, Tris-Cl, pH8.5) within 300-670nm, add 2mg of sodium dithionite powder after scanning, dissolve and reduce After 1 minute, the protein solution was scanned and recorded under the same conditions to determine the binding of heme. For proteins containing heme prosthetic groups, the heme can be replaced by pyridine under alkaline conditions, and the absorption peak of heme disappears at this time. Therefore, another sample was taken in a similar manner, the rhCYGB solution was diluted to about 10nM with 0.2M NaOH and 20% pyridine solution, and the absorption baseline was scanned at 600-500nm. After adding the reducing agent sodium dithionite, the heme absorption peaks of rhCYGB reappeared at 531 and 557nm. This indicates that the refolded and purified rhCYGB molecule contains a heme prosthetic group, see Figure 4 . Use circular dichroism spe...
Embodiment 3
[0028] Alcohol injury was utilized as a model of oxidative stress. Rat liver stellate cells were divided into 1x10 4 / cm 2 Inoculate into 6-well plates, and continue to culture for 24 hours after the cells adhere to the wall. The fresh medium was replaced, and alcohol with a final concentration of 50mM was added to the medium to create a cellular oxidative stress model, and no alcohol was added to the blank group. After adding alcohol for 12 hours, replace the fresh medium and add rhCYGB at final concentrations of 10 μM, 1 μM, and 0.1 μM respectively, and use Tris-Cl solution as a control, and make 6 replicate wells for each concentration. After 12 hours, the medium was aspirated and discarded, and 1ml of PBS buffer was added to blow the cells to make them fall off into a cell suspension. The above-mentioned cell suspension was drawn into a centrifuge tube, and the cells were lysed by ultrasonication (60 Hz, the action time of 2 pulses was 3 seconds each, and the interval w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com