Preparation method of electronic grade high-purity manganese sulfate monohydrate
A technology of monohydrate sulfuric acid and manganese sulfate, applied in manganese sulfate and other directions, can solve the problems of low utilization rate of manganese, strong corrosion of equipment, long process, etc., and achieve the effect of simple production process, low equipment requirements and complete crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
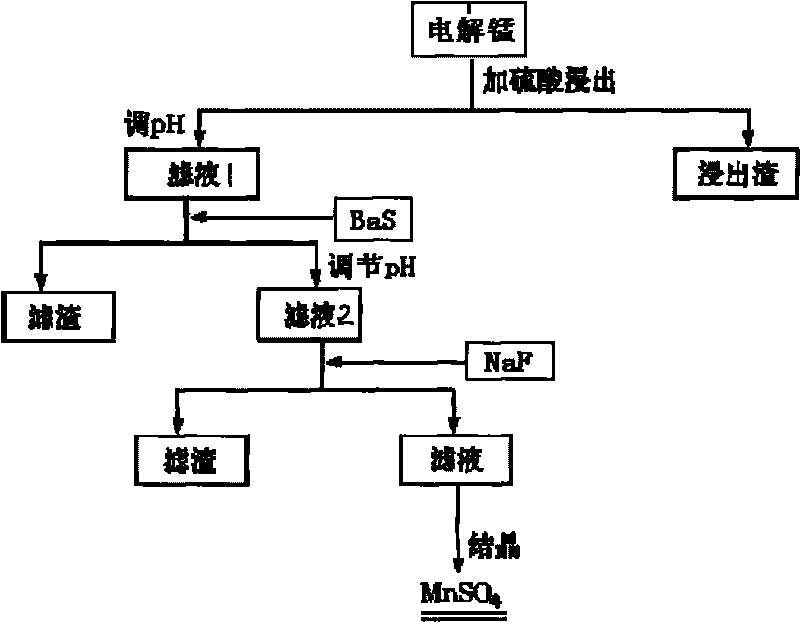
[0021] (1) Grind the electrolytic manganese and pass through a 300-mesh sieve to obtain manganese powder; add the manganese powder to industrial sulfuric acid, heat to 80°C under stirring conditions and continue to react for 12 hours to obtain a manganese sulfate suspension, which is analyzed by atomic absorption The content of the heavy metal salt in the manganese sulfate suspension is determined by spectrum; the mass ratio of the industrial sulfuric acid and manganese powder is 5:1;
[0022] (2) adjust pH to 3 with limestone, add barium sulfide to filter, after reaction 1h, obtain filtrate 1; The quality of described barium sulfide is 2 times of lead salt quality in heavy metal salt;
[0023] (3) add limestone to filtrate 1, adjust the pH value to 6, filter after leaving standstill 1h, obtain filtrate 2;
[0024] (4) Add sodium fluoride to the filtrate 2, filter after reacting for 24 hours, and obtain a manganese sulfate solution; the addition of the sodium fluoride is twice...
Embodiment 2
[0028] (1) Grind the electrolytic manganese and pass through a 350-mesh sieve to obtain manganese powder; add the manganese powder to industrial sulfuric acid, heat it to 90°C under stirring conditions and continue to react for 4 hours to obtain a manganese sulfate suspension, which is analyzed by atomic absorption The content of the heavy metal salt in the manganese sulfate suspension is determined by spectrum; the mass ratio of the industrial sulfuric acid and manganese powder is 4:1;
[0029] (2) adjust pH to 4 with limestone, add barium sulfide to filter, after reacting for 0.5h, obtain filtrate 1; The quality of described barium sulfide is 2 times of lead salt quality in the heavy metal salt;
[0030] (3) add limestone to filtrate 1, adjust the pH value to 6, filter after leaving standstill 1h, obtain filtrate 2;
[0031] (4) Add sodium fluoride to the filtrate 2, filter after reacting for 20 hours, and obtain a manganese sulfate solution; the addition of the sodium fluor...
Embodiment 3
[0035] (1) Grind the electrolytic manganese and pass through a 320-mesh sieve to obtain manganese powder; add the manganese powder to industrial sulfuric acid, heat it to 80°C under stirring conditions and continue to react for 10 hours to obtain a manganese sulfate suspension, which is analyzed by atomic absorption The content of the heavy metal salt in the manganese sulfate suspension is determined by spectrum; the mass ratio of the industrial sulfuric acid and manganese powder is 6:1;
[0036] (2) adjust pH to 3 with limestone, add barium sulfide to filter, after reacting for 0.8h, obtain filtrate 1; The quality of described barium sulfide is 2 times of lead salt quality in heavy metal salt;
[0037] (3) add limestone to filtrate 1, adjust the pH value to 6, filter after leaving standstill 1h, obtain filtrate 2;
[0038] (4) Add sodium fluoride to the filtrate 2, filter after reacting for 20 hours, and obtain a manganese sulfate solution; the addition of the sodium fluoride...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com