Medicament for treating coronary disease and stenocardia and preparation method thereof
A technology for angina pectoris and coronary heart disease, applied in the field of medicine and its preparation, can solve the problems of liquid medicine leakage, complicated production process of soft capsules, and low yield of finished products, so as to improve solubility and absorption, and the production process is simple and controllable , the effect of comprehensive cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
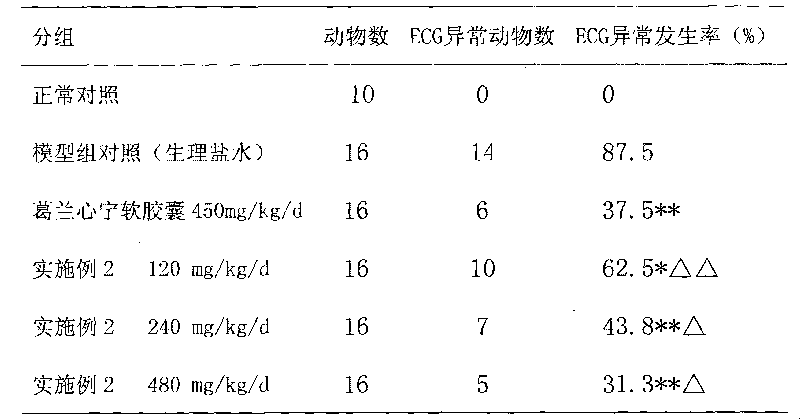
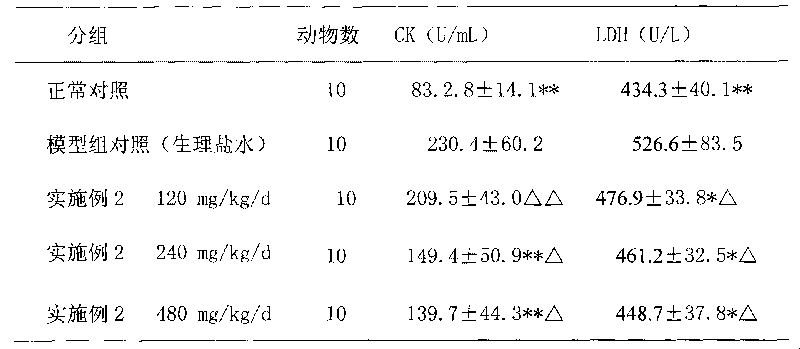
Examples
Embodiment 1
[0022] The present embodiment raw material weight component comprises following medicine:
[0023] 20 parts of total flavonoids of kudzu root, 6 parts of hawthorn extract, 2 parts of gypenosides, 16 parts of hydroxypropyl β-cyclodextrin, 4 parts of calcium hydrogen phosphate, 10 parts of pregelatinized starch, 1.0 parts of croscarmellose sodium part, 0.2 part of micronized silica gel.
[0024] Its raw material weight component of the present embodiment is made up of following medicine:
[0025] 20 parts of total flavonoids of kudzu root, 6 parts of hawthorn extract, 2 parts of gypenosides, 16 parts of hydroxypropyl β-cyclodextrin, 4 parts of calcium hydrogen phosphate, 10 parts of pregelatinized starch, 1.0 parts of croscarmellose sodium part, 0.2 part of micronized silica gel.
[0026] The preparation method steps of the present embodiment are as follows:
[0027] 1. Weigh 6 parts of hawthorn extract with total flavonoids greater than 2.0% based on rutin and a particle siz...
Embodiment 2
[0031] The present embodiment raw material weight component comprises following medicine:
[0032] 20 parts of total flavonoids of kudzu root, 6 parts of hawthorn extract, 2 parts of gypenosides, 20 parts of hydroxypropyl β-cyclodextrin, 6 parts of calcium hydrogen phosphate, 12 parts of pregelatinized starch, 1.4 parts of croscarmellose sodium part, 0.3 part of micronized silica gel.
[0033] Its raw material weight component of the present embodiment is made up of following medicine:
[0034] 20 parts of total flavonoids of kudzu root, 6 parts of hawthorn extract, 2 parts of gypenosides, 20 parts of hydroxypropyl β-cyclodextrin, 6 parts of calcium hydrogen phosphate, 12 parts of pregelatinized starch, 1.4 parts of croscarmellose sodium part, 0.3 part of micronized silica gel.
[0035] The preparation method steps of the present embodiment are as follows:
[0036]1. Weigh 6 parts of hawthorn extract with a total flavonoids greater than 2.0% based on rutin and a particle si...
Embodiment 3
[0040] The present embodiment raw material weight component comprises following medicine:
[0041] 20 parts of total flavonoids of kudzu root, 6 parts of hawthorn extract, 2 parts of gypenosides, 24 parts of hydroxypropyl β-cyclodextrin, 8 parts of calcium hydrogen phosphate, 14 parts of pregelatinized starch, 1.6 parts of croscarmellose sodium part, 0.4 part of micronized silica gel.
[0042] Its raw material weight component of the present embodiment is made up of following medicine:
[0043] 20 parts of total flavonoids of kudzu root, 6 parts of hawthorn extract, 2 parts of gypenosides, 24 parts of hydroxypropyl β-cyclodextrin, 8 parts of calcium hydrogen phosphate, 14 parts of pregelatinized starch, 1.6 parts of croscarmellose sodium part, 0.4 part of micronized silica gel.
[0044] The preparation method steps of the present embodiment are as follows:
[0045] 1. Weigh 6 parts of hawthorn extract with total flavonoids greater than 2.0% based on rutin and a particle siz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com