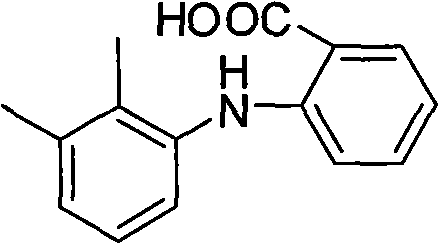
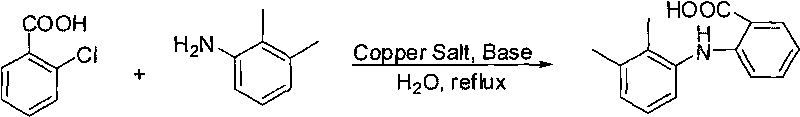Synthesis method of mefenamic acid
A synthetic method, mefenamic acid technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as waste of raw materials, low operation safety, environmental pollution, etc., and reduce production costs and process steps The effect of simplicity and high operational safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 500kg of o-chlorobenzoic acid, 500kg of 2,3-dimethylaniline, 350kg of sodium carbonate, 25kg of anhydrous copper sulfate and 1500kg of water in the reaction vessel, stir evenly, and heat to 100~110°C, React for 15 hours under constant stirring; then dilute the above reaction mixture with water, acidify with dilute hydrochloric acid to PH = 2-3, cool to room temperature after acidification, then filter, and wash the filter residue with warm water at a temperature of 35-40°C to obtain 720kg of formazan An acid crude product.
[0024] Add 720kg of crude mefenamic acid to the reaction vessel, then add 800kg of dimethylformamide to reflux for 3 hours, then cool the solution to room temperature, precipitate solids, then filter, collect the filtered solids into a clean container, and filter to obtain The solid was redissolved in dimethylformamide, heated to reflux for 3 hours, and then cooled to room temperature. A light yellow to white high-purity final product, mefenamic...
Embodiment 2
[0028] Add 500kg of o-chlorobenzoic acid, 400Kg of 2,3-dimethylaniline, 350kg of sodium carbonate, 25kg of anhydrous copper sulfate and 1500kg of water into the reaction vessel, mix well, heat to 90-120°C, and continue The reaction was stirred for 20h. Then the above reaction mixture was diluted with water, acidified with dilute hydrochloric acid to pH=2-3, the acidified reaction solution was cooled to room temperature, then filtered, and the filter residue was washed with warm water to obtain 620 kg of crude mefenamic acid.
[0029] Add 620kg of crude mefenamic acid to the reaction vessel, then add 700kg of acetic acid, stir and react at room temperature for 1 hour, filter and collect a light brown solid, then dissolve the light brown solid in 600kg of dimethylformamide, and heat to reflux After cooling to room temperature for 1 hour, a light yellow to white final product with high purity was precipitated, and dried to constant weight to obtain 490 kg of the final product mef...
Embodiment 3
[0033] Investigate the impact of different feeding ratios of 2,3-dimethylaniline and o-chlorobenzoic acid on the yield of the target product, the results are shown in Table 1:
[0034] Table 1
[0035] label
[0036] Reaction conditions are: sodium carbonate 0.33mol, copper sulfate 0.01mol and water 150g, reaction temperature 90~120 ℃, other reaction conditions are the same as embodiment 1. The results show that when the weight ratio of 2,3-dimethylaniline to o-chlorobenzoic acid is 1:1, that is, when the molar ratio is 1.3:1, the yield of the target product is ideal.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com