Polyene macrolides compound, preparation and application thereof
A kind of macrolide and compound technology, applied in polyene macrolide compound and its preparation and application fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
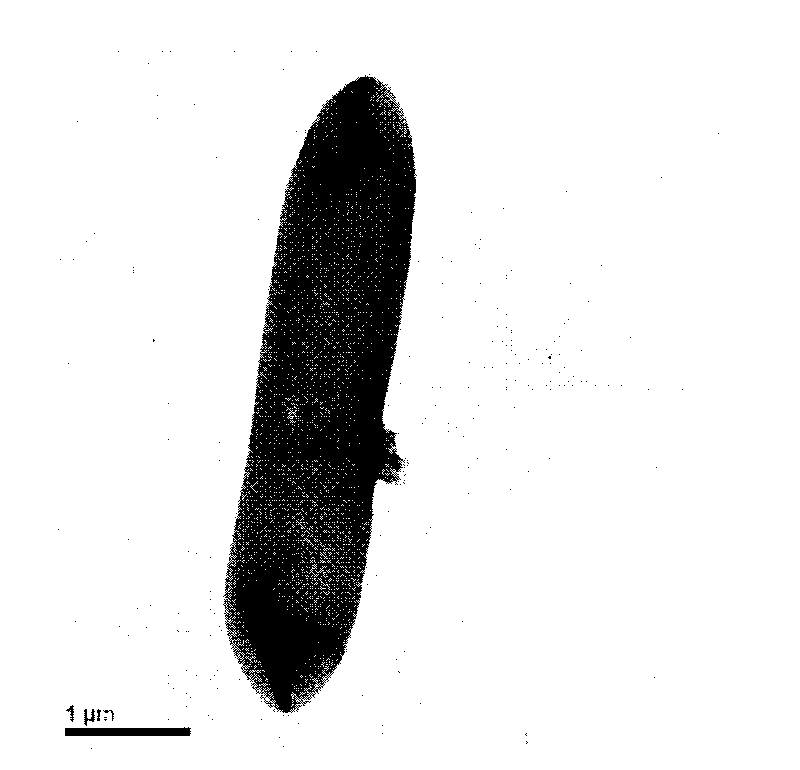
[0060] Example 1: Isolation of Paenibacillus F6-B70
[0061] 1. Collect soil samples at an altitude of 400-1500 meters, and use the dilution coating method to isolate bacterial strains. The specific steps are: weigh 5g of soil samples and add them to a 250ml Erlenmeyer flask filled with 45ml of sterile water, and mix well. Use a pipette to take 1ml and add it to a test tube filled with 9ml sterile water, mix well, and record it as 10 -1 ; take 10 -1 Add 1ml of the solution into a test tube filled with 9ml of sterile water, mix well, and record it as 10 -2 ; Repeatedly diluted in turn until 10 -8 . Take 0.1ml of each dilution gradient and spread evenly on the nutrient agar plate.
[0062] 2. Place the plate at 30°C for 3 days;
[0063] 3. Pick a single colony, dilute it in the same medium and spread it on a plate for culture at 30°C. After repeated separation and purification for three times, check under the microscope and confirm that it is a pure species, pick a purebred...
Embodiment 2
[0065] Embodiment 2: the extraction of Paenibacillus F6-B70 genome
[0066] Referring to the "Guidelines for Molecular Biology Experiments", the details are as follows: the Paenibacillus F6-B70 bacteria obtained in Example 1 were cultured to the late logarithmic growth stage, and 1.5 ml was taken and centrifuged for 2 minutes. Add 567ul of TE buffer, 30ul of 10% SDS and 3ul of 20mg / ml proteinase K to the precipitate, mix well, and incubate at 37°C for 1h. Add 100ul 5mol / L NaCl, 80ul CTAB / NaCl solution (10%CTAB, 0.7M NaCl; preparation method: dissolve 4.1gNaCL in 80ml water, slowly add 10gCTAB, heat to dissolve), mix well, and incubate at 65°C for 1h. Add an equal volume of phenol / chloroform / isoamyl alcohol (25:24:1, v / v / v), mix well, centrifuge for 5 min, transfer the supernatant to a new tube, and repeat this step once. Add 0.6 volume of isopropanol, mix gently until the DNA precipitates, transfer the precipitate to 1 ml of 70% ethanol with a sealed Pasteur tube and wash. C...
Embodiment 3
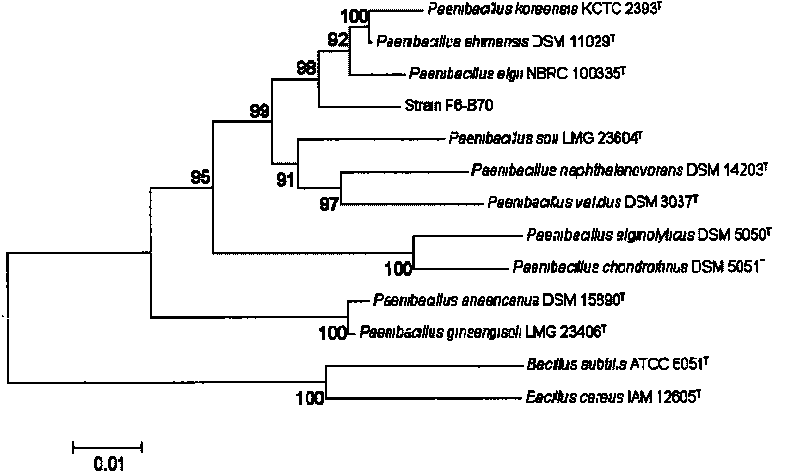
[0067] Embodiment 3: PCR amplification and sequencing of Paenibacillus F6-B70 strain 16S rDNA
[0068]The primers for PCR amplification of 16S rDNA fragments use E8F: 5-AGAGTTTGATCCTGGCTCAG-3 and E1541R: 5-AAGGAGGTGATCCAGCCGCA-3, which can amplify fragments with a length of about 1500bp; the template is the Paenibacillus F6-B70 genome obtained according to Example 2 . Through the optimization of PCR reaction conditions, the optimal PCR conditions and procedures for amplifying 16S rDNA were finally determined: PCR reaction system 20 μl, the specific composition is as follows:
[0069] dNTP (10mM) 2μl
[0070] E8F (20μM) 0.5μl
[0071] E1541R (20μM) 0.5μl
[0072] rTaq (takara) 1.25U
[0073] Template 10ng
[0074] wxya 2 O 12μl
[0075] 10×buffer (for TaKaRa-Taq PCR) 2μl
[0076] The reaction program is: 95°C for 5 min; 95°C for 1 min, 58°C for 1 min, 72°C for 2 min, 30 cycles; 72°C for 12 min.
[0077] The PCR amplification product was purified by electrophoresis firs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com