Self-assembly zinc oxide hollow sphere and preparation method thereof
A technology of zinc oxide and hollow spheres, applied in the direction of zinc oxide/zinc hydroxide, etc., can solve the problems of high cost and energy consumption, expensive production equipment, and difficulty in large-scale production, and achieve low equipment requirements, simple operation, and wide application value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Zinc nitrate hexahydrate and urea raw material are dissolved in the mixed solvent that ethylene glycol and deionized water form, wherein the molar concentration of zinc nitrate hexahydrate and urea is 1: 5, and the volume ratio of ethylene glycol and deionized water is 1:3, fully stirred and dissolved to form Zn 2+ Precursor solution with a molar concentration of 0.1M;
[0026] (2) Put the precursor solution into a closed autoclave, then put the autoclave into an electric heating constant temperature blast drying oven, and perform solvothermal reaction at 110° C. for 6 hours;
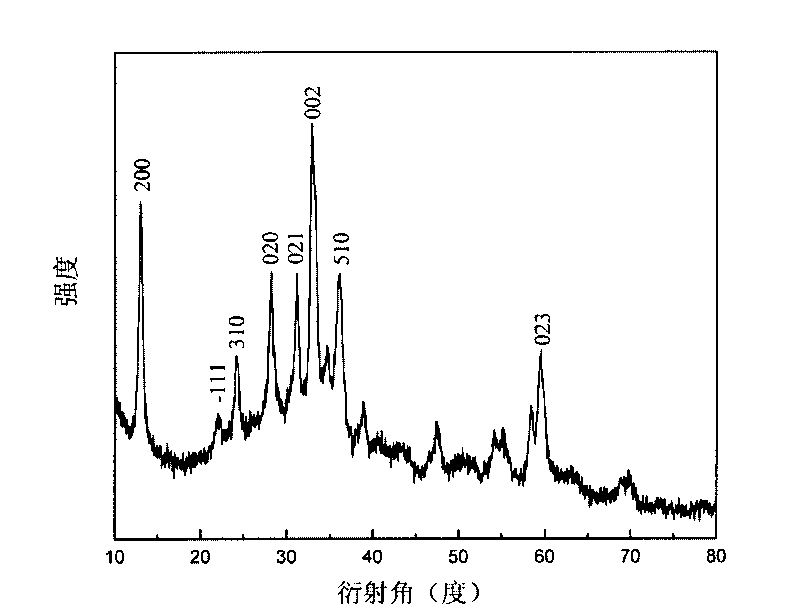
[0027] (3) After the reaction in step (2), cool naturally to room temperature, open the autoclave, filter the reaction product, wash twice with deionized water, then wash twice with absolute ethanol, and dry at 50°C for 3 hours to obtain Basic zinc carbonate precursor.
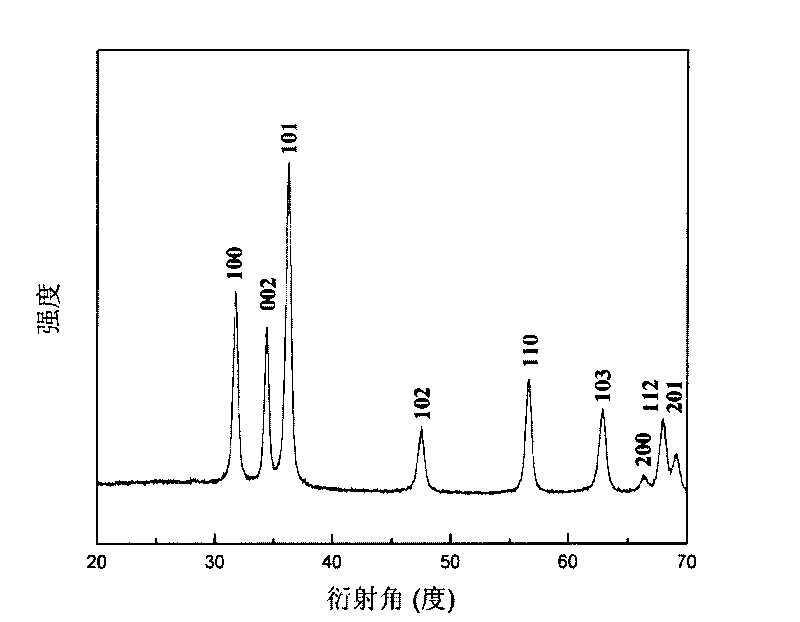
[0028] (4) Put the basic zinc carbonate precursor in a tubular heat treatment furnace, raise the temperature to 300° C. a...
Embodiment 2
[0031] (1) Zinc nitrate hexahydrate and urea raw material are dissolved in the mixed solvent that ethylene glycol and deionized water form, wherein the molar concentration of zinc nitrate hexahydrate and urea is 1: 7, and the volume ratio of ethylene glycol and deionized water is 1:1, fully stirred and dissolved to form Zn 2+ Precursor solution with a molar concentration of 0.2M;
[0032] (2) Put the precursor solution into a closed autoclave, then put the autoclave into an electric heating constant temperature blast drying oven, and perform solvothermal reaction at 120° C. for 9 hours;
[0033] (3) After the reaction in step (2), cool naturally to room temperature, open the autoclave, filter the reaction product, wash twice with deionized water, then wash twice with absolute ethanol, and dry at 55°C for 4 hours to obtain Basic zinc carbonate precursor.
[0034] (4) Put the basic zinc carbonate precursor in a tubular heat treatment furnace, raise the temperature to 350° C. a...
Embodiment 3
[0036](1) Zinc nitrate hexahydrate and urea raw material are dissolved in the mixed solvent that ethylene glycol and deionized water form, wherein the molar concentration of zinc nitrate hexahydrate and urea is 1: 10, and the volume ratio of ethylene glycol and deionized water is 3:1, fully stirred and dissolved to form Zn 2+ Precursor solution with a molar concentration of 0.3M;
[0037] (2) Put the precursor solution into a closed autoclave, then put the autoclave into an electric heating constant temperature blast drying oven, and perform solvothermal reaction at 130°C for 12 hours;
[0038] (3) After the reaction in step (2), cool naturally to room temperature, open the autoclave, filter the reaction product, wash twice with deionized water, then wash twice with absolute ethanol, and dry at 60°C for 5 hours to obtain Basic zinc carbonate precursor.
[0039] (4) Put the basic zinc carbonate precursor in a tubular heat treatment furnace, raise the temperature to 400° C. at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com